Journal of Solid State Chemistry ( IF 3.2 ) Pub Date : 2017-10-03 , DOI: 10.1016/j.jssc.2017.09.032 T. Tiittanen , M. Karppinen
|
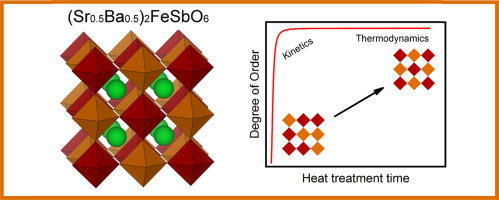
Several sample series of the (Sr0.5Ba0.5)2FeSbO6 double-perovskite compound are synthesized under various heat-treatment conditions starting from a sol-gel prepared precursor to investigate the kinetics and thermodynamics of the B-site cation ordering. The synthesis yields samples with different levels of Fe/Sb ordering ranging from essentially disordered up to fully ordered depending on the synthesis temperature and duration. In the temperature range 1050–1200 °C the degree of order is kinetically controlled following Johnson-Mehl-Avrami-Kolmogorov type kinetics, while at 1300 °C the thermodynamic equilibrium is quickly reached such that higher than 95% ordering degrees are obtained in heat-treatment periods as short as couple of hours. Also importantly, for the differently synthesized single-phase samples a linear relationship between the degree of order and the cubic sub-cell dimension is seen.
中文翻译:

(Sr,Ba)2 FeSbO 6钙钛矿中B位阳离子序的动力学和热力学
从溶胶凝胶制备的前驱体开始,在各种热处理条件下合成了(Sr 0.5 Ba 0.5)2 FeSbO 6双钙钛矿化合物的几个样品系列,以研究B位阳离子排序的动力学和热力学。合成产生的样品具有不同水平的Fe / Sb有序,取决于合成温度和持续时间,从基本无序到完全有序。在1050–1200 °C的温度范围内,有序度是根据Johnson-Mehl-Avrami-Kolmogorov型动力学进行动力学控制的,而在1300℃ °C迅速达到热力学平衡,因此在短短几个小时的热处理时间内可获得高于95%的有序度。同样重要的是,对于不同合成的单相样本,可以看到有序度与立方子电池尺寸之间的线性关系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号