Journal of Solid State Chemistry ( IF 3.2 ) Pub Date : 2017-09-11 , DOI: 10.1016/j.jssc.2017.09.006 Klaus Zöll , Volker Kahlenberg , Hannes Krüger , Peter Tropper
|
In the course of a systematic study of a part of the quaternary system Fe2O3-CaO-Al2O3-MgO (FCAM) the previously unknown compound Ca2.38Mg2.09Fe3+10.61Fe2+1.59Al9.33O36 (FCAM-III) has been synthesized. By analogy with the so-called SFCA series [1–5], our investigation in the system of FCAM shows the existence of a stoichiometric homologous series M14+6nO20+8n, where M = Fe, Ca, Al, Mg and n = 1 or 2.
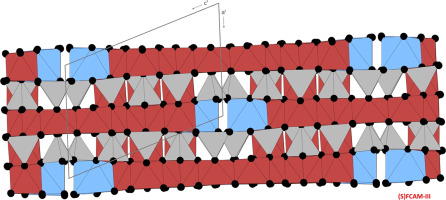
In air, we can prove the formation of coexisting FCAM-III and FCAM-I solid solutions at 1400 °C. By increasing the temperature up to 1425 °C FCAM-I disappears completely and FCAM-III co-exists with magnesiumferrite and a variety of calcium iron oxides. At 1450 °C FCAM-III breaks down to a mixture of FCAM-I again as well as magnesioferrite and melt. Small single-crystals of FCAM-III up to 35 µm in size could be retrieved from the 1425 °C experiment and were subsequently characterized using electron microprobe analysis and synchroton X-ray single-crystal diffraction. Finally the Fe2+/Fetot ratio was calculated from the total iron content based on the crystal-chemical formula obtained from EMPA measurements and charge balance considerations. FCAM-III or Ca2.38Mg2.09Fe3+10.61Fe2+1.59Al9.33O36 has a triclinic crystal structure (space group P ). The basic crystallographic data are: a = 10.223(22) Å, b = 10.316(21) Å, c = 14.203(15) Å, α = 93.473(50)°, β = 107.418(67)°, γ = 109.646(60)°, V = 1323.85(2) ų, Z = 1.
Using Schreinemaker's technique to analyze the phase relations in the system Fe2O3-CaO-Al2O3-MgO it was possible to obtain the semi-quantitative stability relations between the participating phases and construct a topologically correct phase sequence as a function of T and fO2. The analysis shows that Ca2Al0.5Fe1.5O5 (C2A0.25F0.75) and CaAl1.5Fe2.5O7 (CA0.75F1.25) with higher calculated Fe2+ contents are preferably formed at lower oxygen fugacity and react to CaAl0.5Fe1.5O4 (CA0.25F0.75) by increasing fO2. Spinel-type magnesium-aluminium-ferrite (Mg,Fe2+)Fe3+1.25Al0.75O4 or (MA0.375F0.625) is the typical phase which occurs at high temperature (1400 °C).
中文翻译:

FCAM-III(Ca 2.38 Mg 2.09 Fe 3+ 10.61 Fe 2+ 1.59 Al 9.33 O 36)的研究:CaO-MgO-Fe 2 O 3 -Al 2 O 3系统中铜镁矿结构型的新同系物
在对部分四元体系Fe 2 O 3 -CaO-Al 2 O 3 -MgO(FCAM)进行系统研究的过程中,先前未知的化合物Ca 2.38 Mg 2.09 Fe 3+ 10.61 Fe 2+ 1.59 Al 9.33 O 36(FCAM-III)已经合成。通过类似于所谓的SFCA系列[1-5],我们在FCAM系统中的研究表明存在化学计量的同源序列M 14 + 6n O 20 + 8n,其中M = Fe,Ca,Al,Mg和n = 1或2。
在空气中,我们可以证明在1400 °C下共存的FCAM-III和FCAM-I固溶体的形成。通过将温度升高至1425 °C,FCAM-I完全消失,FCAM-III与亚铁酸镁和多种钙铁氧化物共存。在1450 °C时,FCAM-III再次分解为FCAM-I的混合物,以及镁铁矿和熔体。 可以从1425 °C实验中回收尺寸最大为35 µm的FCAM-III小单晶,随后使用电子微探针分析和同步X射线单晶衍射进行表征。最后是Fe 2+ / Fe tot根据总铁含量,根据从EMPA测量获得的晶体化学公式和电荷平衡考虑因素,计算出铁的比例。FCAM-III或Ca 2.38 Mg 2.09 Fe 3+ 10.61 Fe 2+ 1.59 Al 9.33 O 36具有三斜晶体结构(空间群P )。基本晶体学数据为:a = 10.223(22)Å,b = 10.316(21)Å,c = 14.203(15)Å,α= 93.473(50)°,β= 107.418(67)°,γ= 109.646( 60)°,V = 1323.85(2)ų,Z = 1。
使用Schreinemaker的技术分析Fe 2 O 3 -CaO-Al 2 O 3 -MgO系统中的相关系,可以获得参与相之间的半定量稳定性关系,并构造一个拓扑正确的相序作为函数。T和f O 2。分析表明,Ca 2 Al 0.5 Fe 1.5 O 5(C 2 A 0.25 F 0.75)和CaAl 1.5 Fe 2.5 O 7(CA 0.75 F 1.25优选地,在较低的氧逸度下形成具有较高计算的Fe 2+含量的Fe 2+),并通过增加f O 2与CaAl 0.5 Fe 1.5 O 4(CA 0.25 F 0.75)反应。尖晶石型镁铝铁氧体(Mg,Fe 2+)Fe 3+ 1.25 Al 0.75 O 4或(MA 0.375 F 0.625)是在高温(1400 °C)下出现的典型相。











































 京公网安备 11010802027423号
京公网安备 11010802027423号