Tetrahedron ( IF 2.1 ) Pub Date : 2017-12-15 , DOI: 10.1016/j.tet.2017.12.027 Lin You , Chuo Chen
|
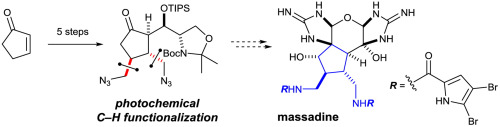
The ability of triplet ketones to abstract a hydrogen atom from hydrocarbons is reminiscent of that of the high-spin metal-oxo complexes in C–H oxidation enzymes. In practice, the reactivity of triplet ketones is easier to control and applicable to promoting a wider range of reactions. We demonstrate herein the synthetic utility of triplet ketone-mediated C-addition of methanol to cyclopentenone derivatives with an expedient synthesis of the core skeleton of the [3 + 2]-type dimeric pyrrole–imidazole alkaloids. Remarkably, this photochemical C–H functionalization reaction is highly regioselective and can tolerate a good range of functional groups.
中文翻译:

通过三重酮介导的C–H功能化,可快速访问[3 + 2]型二聚吡咯-咪唑生物碱的核心骨架
三重态酮从碳氢化合物中提取氢原子的能力让人联想到CH-H氧化酶中的高自旋金属-氧代配合物。在实践中,三线态酮的反应性更易于控制,可用于促进更广泛的反应。我们在本文中证明了三重态酮介导的甲醇向环戊烯酮衍生物的C加成的合成效用,以及[3 + 2]型二聚吡咯-咪唑生物碱的核心骨架的方便合成。值得注意的是,这种光化学C–H官能化反应具有很高的区域选择性,并且可以耐受各种官能团。











































 京公网安备 11010802027423号
京公网安备 11010802027423号