Tetrahedron ( IF 2.1 ) Pub Date : 2017-12-15 , DOI: 10.1016/j.tet.2017.12.026 Jonathan M.E. Hughes , James L. Gleason
|
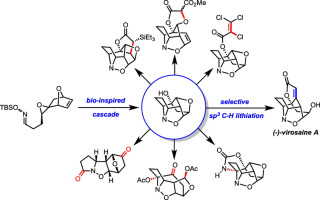
The asymmetric total synthesis of (−)-virosaine A was achieved in 9% overall yield from commercially/readily available starting materials. Inspired by an intriguing biosynthetic proposal, a novel cascade reaction sequence was developed to efficiently construct the caged polycyclic core of virosaine A. The pivotal cascade precursor was readily available in enantiopure form via a robust route that featured an enantioselective one-pot Diels-Alder cycloaddition/organolithium addition. Several contemporary methods of CH functionalization were applied to the cascade product and yielded a diverse set of novel complex polycycles. Ultimately, a combination of NMR and computational analyses laid the groundwork for a successful directed lithiation strategy to selectively functionalize the caged core and complete the total synthesis of virosaine A.
中文翻译:

生物启发的级联和后期定向的sp 3 C
(-)-virosaine A的不对称全合成是从市售/容易获得的起始原料中实现的,总产率为9%。受一个有趣的生物合成提议的启发,开发了一种新型的级联反应序列,以有效地构建virosaine A的笼状多环核心。关键的级联前体很容易通过对映纯形式通过对映选择性单锅Diels-Alder环加成的健壮途径获得。/ organolithium添加。C的几种当代方法H功能化应用于级联产物,并产生了多种多样的新型复杂多环化合物。最终,将NMR和计算分析相结合,为成功的定向锂化策略奠定了基础,该策略可选择性地使笼状核官能化并完成virosaine A的总合成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号