当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Improved Biopharmaceutical Properties of Oral Formulations of 1,2,4-Thiadiazole Derivative with Cyclodextrins: in Vitro and in Vivo Evaluation
ACS Biomaterials Science & Engineering ( IF 5.8 ) Pub Date : 2018-01-03 00:00:00 , DOI: 10.1021/acsbiomaterials.7b00887 Maria Promzeleva 1 , Tatyana Volkova 1 , Alexey Proshin 2 , Oleg Siluykov 1, 3 , Anton Mazur 3 , Peter Tolstoy 3 , Sergey Ivanov 4 , Felix Kamilov 5 , Irina Terekhova 1
ACS Biomaterials Science & Engineering ( IF 5.8 ) Pub Date : 2018-01-03 00:00:00 , DOI: 10.1021/acsbiomaterials.7b00887 Maria Promzeleva 1 , Tatyana Volkova 1 , Alexey Proshin 2 , Oleg Siluykov 1, 3 , Anton Mazur 3 , Peter Tolstoy 3 , Sergey Ivanov 4 , Felix Kamilov 5 , Irina Terekhova 1
Affiliation
|
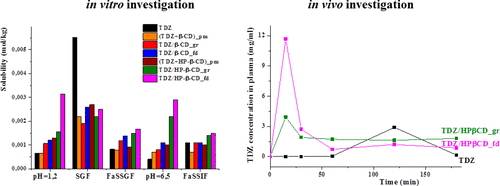
The synthesized 1,2,4-thiadiazole derivative displaying biological activity has low aqueous solubility and dissolution rate. Novel oral formulations of thiadiazole with β- and hydroxypropyl-β-cyclodextrins were obtained by grinding and freeze-drying methods with the purpose to improve the aqueous solubility. Complex formation of 1,2,4-thiadiazole derivative with cyclodextrins was confirmed by means of solid-state 13C MAS CP/TOSS NMR. Solubility, dissolution rate and permeability of the solid inclusion complexes were evaluated in different biorelevant media (SGF, FaSSGF, FaSSIF) simulating the conditions in the gastrointestinal tract. It was demonstrated that the content of biorelevant media affects the properties of the inclusion complexes. In particular, solubilizing effect of cyclodextrins became less pronounced when the micelles of taurocholic acid and lecithin are formed in the dissolution media. The inclusion of thiadiazole into cyclodextrin cavity is in competition with its partitioning into the micelles and this should be taken into account when the in vivo behavior is predicted. The results of in vitro and in vivo experiments were found to be in agreement and showed the highest solubility, dissolution rate and bioavailability of the freeze-dried complexes of thiadiazole with hydroxypropyl-β-cyclodextrin. These complexes can be proposed as more effective dosage forms for oral administration.
中文翻译:

1,2,4-噻二唑衍生物与环糊精的口服制剂的改进生物药物特性:体内和体外评估
合成的具有生物活性的1,2,4-噻二唑衍生物具有较低的水溶性和溶解速率。通过研磨和冷冻干燥方法获得了噻二唑与β-和羟丙基-β-环糊精的新型口服制剂,目的是提高水溶性。固态13证实了1,2,4-噻二唑衍生物与环糊精的复杂形成C MAS CP / TOSS NMR。在模拟胃肠道状况的不同生物相关介质(SGF,FaSSGF,FaSSIF)中评估了固体包合物的溶解度,溶解速率和渗透性。已证明生物相关介质的含量影响包合物的性质。特别地,当牛磺胆酸和卵磷脂的胶束在溶解介质中形成时,环糊精的增溶作用变得不太明显。噻二唑在环糊精腔中的存在与其在胶束中的分配竞争,并且在预测体内行为时应考虑到这一点。体外和体内实验的结果一致,显示出最高的溶解度,噻二唑与羟丙基-β-环糊精的冻干复合物的溶出度和生物利用度。这些复合物可以作为口服给药的更有效剂型被提出。
更新日期:2018-01-03
中文翻译:

1,2,4-噻二唑衍生物与环糊精的口服制剂的改进生物药物特性:体内和体外评估
合成的具有生物活性的1,2,4-噻二唑衍生物具有较低的水溶性和溶解速率。通过研磨和冷冻干燥方法获得了噻二唑与β-和羟丙基-β-环糊精的新型口服制剂,目的是提高水溶性。固态13证实了1,2,4-噻二唑衍生物与环糊精的复杂形成C MAS CP / TOSS NMR。在模拟胃肠道状况的不同生物相关介质(SGF,FaSSGF,FaSSIF)中评估了固体包合物的溶解度,溶解速率和渗透性。已证明生物相关介质的含量影响包合物的性质。特别地,当牛磺胆酸和卵磷脂的胶束在溶解介质中形成时,环糊精的增溶作用变得不太明显。噻二唑在环糊精腔中的存在与其在胶束中的分配竞争,并且在预测体内行为时应考虑到这一点。体外和体内实验的结果一致,显示出最高的溶解度,噻二唑与羟丙基-β-环糊精的冻干复合物的溶出度和生物利用度。这些复合物可以作为口服给药的更有效剂型被提出。



























 京公网安备 11010802027423号
京公网安备 11010802027423号