当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Insights into Aerobic Oxidative Methyl Esterification of Primary Alcohols with Heterogeneous PdBiTe Catalysts
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1021/acscatal.7b02886 David S. Mannel 1 , Jesaiah King 1 , Yuliya Preger 1 , Maaz S. Ahmed 1 , Thatcher W. Root 1 , Shannon S. Stahl 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1021/acscatal.7b02886 David S. Mannel 1 , Jesaiah King 1 , Yuliya Preger 1 , Maaz S. Ahmed 1 , Thatcher W. Root 1 , Shannon S. Stahl 1
Affiliation
|
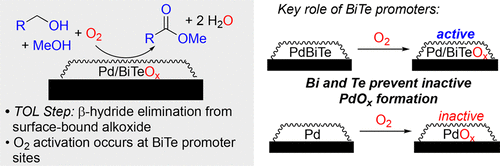
Aerobic oxidative methyl esterification of primary alcohols is an important chemical transformation that converts a nucleophile (alcohol) into a versatile electrophile (methyl ester). We recently discovered a heterogeneous PdBiTe/C catalyst that exhibits the highest activity yet reported for this transformation. Bi and Te serve as synergistic promoters that enhance both the rate and yield of the reactions relative to reactions employing Pd alone or Pd in combination with Bi or with Te as the sole promoter. Here, we report a mechanistic study of the oxidative methyl esterification of benzyl alcohol and 1-octanol to provide insights into the overall multistep transformation as well as the role of the Bi and Te in the reaction. The catalytic rates of the oxidative esterification of benzyl alcohol and octanol with Pd, PdBi, PdTe, and PdBiTe catalysts exhibit a saturation dependence on [alcohol] and [K2CO3] and a first-order dependence on pO2. Hammett studies of benzyl alcohol oxidation reveal opposing electronic trends for initial rates of oxidation of alcohol to aldehyde (negative ρ value) and the oxidation of aldehyde to methyl ester (positive ρ value). These data and complementary kinetic isotope effect data support a Langmuir–Hinshelwood mechanism in which a surface-bound alkoxide or hemiacetal intermediate undergoes rate-limiting β-hydride elimination. Molecular oxygen participates in this process, as revealed by a first-order dependence on pO2. X-ray photoelectron and X-ray absorption spectroscopic methods show that the promoters undergo oxidation in preference to Pd, maintaining the Pd surface in the active metallic state and preventing inhibition by surface Pd-oxide formation. Collectively, these results provide valuable insights into the synergistic benefits of multiple promoters in heterogeneous catalytic oxidation reactions.
中文翻译:

用多相PdBiTe催化剂对伯醇进行好氧氧化甲基酯化的机理研究
伯醇的好氧氧化甲基酯化是一种重要的化学转化,它将亲核试剂(醇)转化为通用的亲电试剂(甲酯)。我们最近发现了一种非均相的PdBiTe / C催化剂,该催化剂显示出迄今报道的最高转化活性。Bi和Te用作协同促进剂,相对于单独使用Pd或将Pd与Bi或与Te或Te作为唯一促进剂结合使用的反应而言,Bi和Te可以提高反应的速率和产率。在这里,我们报告了对苄醇和1-辛醇的氧化甲基酯化的机理研究,以提供对整体多步转化以及Bi和Te在反应中的作用的了解。苯甲醇和辛醇与Pd,PdBi,PdTe,2 CO 3 ]和对p O 2的一阶依赖性。Hammett对苯甲醇氧化的研究揭示了醇氧化为醛的初始速率(负ρ值)和醛氧化为甲酯(正ρ值)的相反电子趋势。这些数据和互补的动力学同位素效应数据支持Langmuir-Hinshelwood机理,在该机理中,表面结合的醇盐或半缩醛中间体经历了限速β-氢化物消除。分子氧参与该过程,如对p O 2的一阶依赖性所揭示的。X射线光电子和X射线吸收光谱法显示,助催化剂优先于Pd进行氧化,将Pd表面保持在活性金属状态,并防止表面Pd-氧化物形成的抑制。总的来说,这些结果为异质催化氧化反应中多个启动子的协同效益提供了有价值的见解。
更新日期:2018-01-09
中文翻译:

用多相PdBiTe催化剂对伯醇进行好氧氧化甲基酯化的机理研究
伯醇的好氧氧化甲基酯化是一种重要的化学转化,它将亲核试剂(醇)转化为通用的亲电试剂(甲酯)。我们最近发现了一种非均相的PdBiTe / C催化剂,该催化剂显示出迄今报道的最高转化活性。Bi和Te用作协同促进剂,相对于单独使用Pd或将Pd与Bi或与Te或Te作为唯一促进剂结合使用的反应而言,Bi和Te可以提高反应的速率和产率。在这里,我们报告了对苄醇和1-辛醇的氧化甲基酯化的机理研究,以提供对整体多步转化以及Bi和Te在反应中的作用的了解。苯甲醇和辛醇与Pd,PdBi,PdTe,2 CO 3 ]和对p O 2的一阶依赖性。Hammett对苯甲醇氧化的研究揭示了醇氧化为醛的初始速率(负ρ值)和醛氧化为甲酯(正ρ值)的相反电子趋势。这些数据和互补的动力学同位素效应数据支持Langmuir-Hinshelwood机理,在该机理中,表面结合的醇盐或半缩醛中间体经历了限速β-氢化物消除。分子氧参与该过程,如对p O 2的一阶依赖性所揭示的。X射线光电子和X射线吸收光谱法显示,助催化剂优先于Pd进行氧化,将Pd表面保持在活性金属状态,并防止表面Pd-氧化物形成的抑制。总的来说,这些结果为异质催化氧化反应中多个启动子的协同效益提供了有价值的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号