当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cocrystals Help Break the “Rules” of Isostructurality: Solid Solutions and Polymorphism in the Malic/Tartaric Acid System
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2017-12-27 00:00:00 , DOI: 10.1021/acs.cgd.7b01321 Aurora J. Cruz-Cabeza 1 , Monica Lestari 2 , Matteo Lusi 2
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2017-12-27 00:00:00 , DOI: 10.1021/acs.cgd.7b01321 Aurora J. Cruz-Cabeza 1 , Monica Lestari 2 , Matteo Lusi 2
Affiliation
|
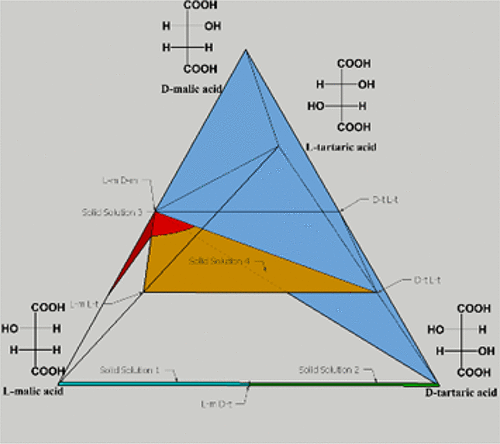
Crystalline solid solutions have the potential to afford tunable materials for pharmaceutical and technological applications. Unfortunately, these poorly understood phases are difficult to obtain and, hence, to study. In fact, commonly accepted empirical rules prescribe that only molecules of similar size and electron distribution are mutually soluble in the solid state. Here, despite the evident structural and electronic differences, the enantiomers of malic acid and tartaric acid are crystallized together in a variable stoichiometric ratio to produce both cocrystals and solid solutions. In some cases, physical mixtures are observed. The composition and polymorphism of the crystalline products are explained by DFT-d molecular substitution calculations for the cocrystallized molecules in different (known) structures. At the same time, from a crystal engineering perspective, the behavior of this complex system is rationalized thanks to the existence of intermediate cocrystal forms that merge the structural features of the pure molecular components.
中文翻译:

共晶体有助于打破同构性的“规则”:苹果酸/酒石酸体系中的固溶体和多态性
结晶固溶体具有为制药和技术应用提供可调谐材料的潜力。不幸的是,这些难以理解的阶段很难获得,因此难以研究。实际上,普遍接受的经验规则规定,只有大小和电子分布相似的分子才能互溶成固态。在此,尽管存在明显的结构和电子差异,但苹果酸和酒石酸的对映异构体以可变的化学计量比一起结晶,从而产生共晶体和固溶体。在某些情况下,观察到物理混合物。结晶产物的组成和多态性通过不同(已知)结构的共结晶分子的DFT-d分子取代计算来解释。同时,
更新日期:2017-12-27
中文翻译:

共晶体有助于打破同构性的“规则”:苹果酸/酒石酸体系中的固溶体和多态性
结晶固溶体具有为制药和技术应用提供可调谐材料的潜力。不幸的是,这些难以理解的阶段很难获得,因此难以研究。实际上,普遍接受的经验规则规定,只有大小和电子分布相似的分子才能互溶成固态。在此,尽管存在明显的结构和电子差异,但苹果酸和酒石酸的对映异构体以可变的化学计量比一起结晶,从而产生共晶体和固溶体。在某些情况下,观察到物理混合物。结晶产物的组成和多态性通过不同(已知)结构的共结晶分子的DFT-d分子取代计算来解释。同时,











































 京公网安备 11010802027423号
京公网安备 11010802027423号