Catalysis Today ( IF 5.2 ) Pub Date : 2017-12-15 , DOI: 10.1016/j.cattod.2017.12.004 Yanan Yuan , Dan Zhao , Jinjun Li , Feng Wu , Marcello Brigante , Gilles Mailhot
|
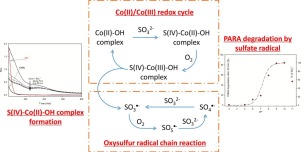
In this study we have investigated the efficiency Cobalt (II) (Co(II)) for the activation of sulfite ions following the oxidation of paracetamol used as model contaminants. Physico-chemical parameters that can impact the paracetamol degradation (pH, initial paracetamol concentration, Co(II)/S(IV) molar ratio, oxygen concentration) and contribution of various radicals were investigated in order to elucidate the chemical mechanism. Main results show that the pH is a key factor controlling the efficiency in the system Co(II)/Sulfite. Higher efficiency is observed for pH between 9.0 and 10.0. Increasing S(IV) concentrations, until 1 mM, slightly promoted the degradation of paracetamol. In fact, an excess of sulfite ions inhibits the reaction through the scavenging of SO4− and SO5
−. Moreover, degradation efficiency drastically decreases from ∼ 85% to less than 5% in absence of oxygen. SO4
− was confirmed to be the main oxidant responsible for the paracetamol degradation. For the first time we determined the second order rate constant between SO4
− and paracetamol (1.33 ± 0.79 × 109 M−1 s−1 (at pH 5) and 6.14 ± 0.99 × 108 M−1 s−1 (at pH 11.0)). Moreover, radical-scavenging experiments also suggest the possible implication of SO5
−. Hence, this work provides a precise understanding of the overall mechanism and a new promising strategy by using sulfite and transition metal such as Co(II) to promote organic compounds degradation in water under neutral and alkaline pH conditions.
中文翻译:

钴(II)催化的亚硫酸盐在碱性pH下快速氧化对乙酰氨基酚
在这项研究中,我们研究了用作模型污染物的扑热息痛氧化后,钴(II)(Co(II))活化亚硫酸根离子的效率。为了阐明化学机理,研究了可能影响扑热息痛降解的理化参数(pH,扑热息痛初始浓度,Co(II)/ S(IV)摩尔比,氧浓度)和各种自由基的贡献。主要结果表明,pH是控制Co(II)/亚硫酸盐体系效率的关键因素。在9.0和10.0之间的pH值下观察到更高的效率。增加S(IV)浓度直至1 mM会轻微促进对乙酰氨基酚的降解。实际上,过量的亚硫酸根离子抑制通过SO的清除反应4 -和SO 5
-。而且,在没有氧气的情况下,降解效率从约85%急剧降低到5%以下。SO 4
-被证实为负责的对乙酰氨基酚降解的主要氧化剂。首次我们测定SO之间的二级速率常数4
-和对乙酰氨基酚(1.33±0.79×10 9 中号-1 小号-1(在pH 5)和6.14±0.99×10 8 中号-1 小号-1(在pH 11.0))。此外,清除自由基的实验也表明SO的可能产生的影响5
-。因此,这项工作通过使用亚硫酸盐和过渡金属(例如Co(II))来促进有机化合物在中性和碱性pH条件下在水中的降解,从而提供了对整体机理的精确理解和新的有希望的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号