当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multifunctional Protocells for Enhanced Penetration in 3D Extracellular Tumoral Matrices
Chemistry of Materials ( IF 7.2 ) Pub Date : 2017-12-26 00:00:00 , DOI: 10.1021/acs.chemmater.7b03128 María Rocío Villegas 1, 2 , Alejandro Baeza 1, 2 , Achraf Noureddine , Paul N. Durfee , Kimberly S. Butler , Jacob Ongudi Agola , C. Jeffrey Brinker , María Vallet-Regí 1, 2
Chemistry of Materials ( IF 7.2 ) Pub Date : 2017-12-26 00:00:00 , DOI: 10.1021/acs.chemmater.7b03128 María Rocío Villegas 1, 2 , Alejandro Baeza 1, 2 , Achraf Noureddine , Paul N. Durfee , Kimberly S. Butler , Jacob Ongudi Agola , C. Jeffrey Brinker , María Vallet-Regí 1, 2
Affiliation
|
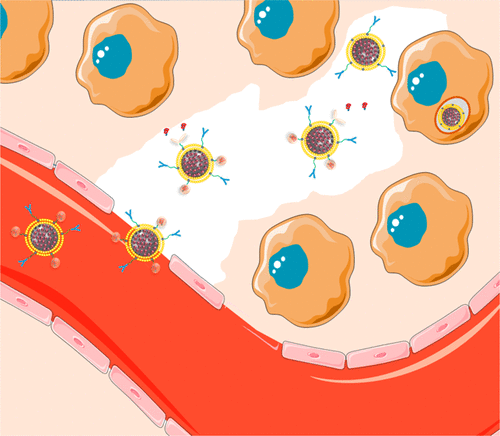
The high density of the extracellular matrix in solid tumors is an important obstacle to nanocarriers for reaching deep tumor regions and has severely limited the efficacy of administrated nanotherapeutics. The use of proteolytic enzymes prior to nanoparticle administration or directly attached to the nanocarrier surface has been proposed to enhance their penetration, but the low in vivo stability of these macromolecules compromises their efficacy and strongly limits their application. Herein, we have designed a multifunctional nanocarrier able to transport cytotoxic drugs to deep areas of solid tumors and once there, to be engulfed by tumoral cells causing their destruction. This system is based on mesoporous silica nanocarriers encapsulated within supported lipid bilayers (SLBs). The SLB avoids premature release of the housed drug while providing high colloidal stability and an easy to functionalize surface. The tumor penetration property is provided by attachment of engineered polymeric nanocapsules that transport and controllably unveil and release the proteolytic enzymes that in turn digest the extracellular matrix, facilitating the nanocarrier diffusion through the matrix. Additionally, targeting properties were endowed by conjugating an antibody specific to the investigated tumoral cells to enhance binding, internalization, and drug delivery. This multifunctional design improves the therapeutic efficacy of the transported drug as a consequence of its more homogeneous distribution throughout the tumoral tissue.
中文翻译:

增强3D细胞外基质渗透性的多功能原细胞
实体瘤中细胞外基质的高密度是纳米载体到达深部肿瘤区域的重要障碍,并严重限制了纳米治疗药物的疗效。已经提出在施用纳米颗粒之前或直接附着在纳米载体表面上使用蛋白水解酶来增强其渗透性,但体内渗透率低。这些大分子的稳定性损害了它们的功效,并严重限制了它们的应用。本文中,我们设计了一种多功能纳米载体,能够将细胞毒性药物转运至实体瘤的深层区域,一旦到达该区域,就会被肿瘤细胞吞噬,从而导致其破坏。该系统基于包封在支持的脂质双层(SLB)中的中孔二氧化硅纳米载体。SLB避免了所容纳药物的过早释放,同时提供了高的胶体稳定性和易于功能化的表面。肿瘤穿透特性是通过附着工程化的聚合物纳米胶囊来提供的,该聚合物纳米粒运输并可控制地揭示和释放蛋白水解酶,进而分解细胞外基质,从而促进纳米载体通过基质的扩散。此外,通过缀合对所研究的肿瘤细胞具有特异性的抗体来增强靶向性,以增强结合,内在化和药物传递。由于其在整个肿瘤组织中分布更均匀,因此这种多功能设计提高了所运输药物的治疗功效。
更新日期:2017-12-26
中文翻译:

增强3D细胞外基质渗透性的多功能原细胞
实体瘤中细胞外基质的高密度是纳米载体到达深部肿瘤区域的重要障碍,并严重限制了纳米治疗药物的疗效。已经提出在施用纳米颗粒之前或直接附着在纳米载体表面上使用蛋白水解酶来增强其渗透性,但体内渗透率低。这些大分子的稳定性损害了它们的功效,并严重限制了它们的应用。本文中,我们设计了一种多功能纳米载体,能够将细胞毒性药物转运至实体瘤的深层区域,一旦到达该区域,就会被肿瘤细胞吞噬,从而导致其破坏。该系统基于包封在支持的脂质双层(SLB)中的中孔二氧化硅纳米载体。SLB避免了所容纳药物的过早释放,同时提供了高的胶体稳定性和易于功能化的表面。肿瘤穿透特性是通过附着工程化的聚合物纳米胶囊来提供的,该聚合物纳米粒运输并可控制地揭示和释放蛋白水解酶,进而分解细胞外基质,从而促进纳米载体通过基质的扩散。此外,通过缀合对所研究的肿瘤细胞具有特异性的抗体来增强靶向性,以增强结合,内在化和药物传递。由于其在整个肿瘤组织中分布更均匀,因此这种多功能设计提高了所运输药物的治疗功效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号