当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reshaping the Energy Landscape Transforms the Mechanism and Binding Kinetics of DNA Threading Intercalation
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-15 00:00:00 , DOI: 10.1021/acs.biochem.7b01036 Andrew G. Clark 1 , M. Nabuan Naufer 1 , Fredrik Westerlund 2 , Per Lincoln 3 , Ioulia Rouzina 4 , Thayaparan Paramanathan 5 , Mark C. Williams 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-15 00:00:00 , DOI: 10.1021/acs.biochem.7b01036 Andrew G. Clark 1 , M. Nabuan Naufer 1 , Fredrik Westerlund 2 , Per Lincoln 3 , Ioulia Rouzina 4 , Thayaparan Paramanathan 5 , Mark C. Williams 1
Affiliation
|
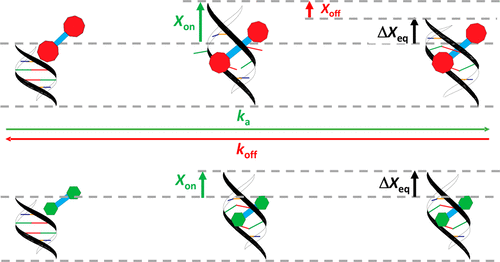
Molecules that bind DNA via threading intercalation show high binding affinity as well as slow dissociation kinetics, properties ideal for the development of anticancer drugs. To this end, it is critical to identify the specific molecular characteristics of threading intercalators that result in optimal DNA interactions. Using single-molecule techniques, we quantify the binding of a small metal–organic ruthenium threading intercalator (Δ,Δ-B) and compare its binding characteristics to a similar molecule with significantly larger threading moieties (Δ,Δ-P). The binding affinities of the two molecules are the same, while comparison of the binding kinetics reveals significantly faster kinetics for Δ,Δ-B. However, the kinetics is still much slower than that observed for conventional intercalators. Comparison of the two threading intercalators shows that the binding affinity is modulated independently by the intercalating section and the binding kinetics is modulated by the threading moiety. In order to thread DNA, Δ,Δ-P requires a “lock mechanism”, in which a large length increase of the DNA duplex is required for both association and dissociation. In contrast, measurements of the force-dependent binding kinetics show that Δ,Δ-B requires a large DNA length increase for association but no length increase for dissociation from DNA. This contrasts strongly with conventional intercalators, for which almost no DNA length change is required for association but a large DNA length change must occur for dissociation. This result illustrates the fundamentally different mechanism of threading intercalation compared with conventional intercalation and will pave the way for the rational design of therapeutic drugs based on DNA threading intercalation.
中文翻译:

重塑能量格局改变了DNA穿插的机理和结合动力学
通过穿线插入结合DNA的分子显示出高的结合亲和力和缓慢的解离动力学,这是开发抗癌药物的理想特性。为此,至关重要的是要确定导致最佳DNA相互作用的穿线嵌入剂的特定分子特征。使用单分子技术,我们可以量化小金属-有机钌穿线嵌入剂(Δ,Δ- B)的结合,并比较其与具有明显更大穿线部分(Δ,Δ- P)的相似分子的结合特性。两个分子的结合亲和力相同,而结合动力学的比较表明,Δ,Δ- B的动力学明显更快。。然而,动力学仍然比常规嵌入剂观察到的慢得多。两种穿线嵌入剂的比较表明,结合亲和力由嵌入部分独立调节,而结合动力学由穿线部分调节。为了穿入DNA,Δ,Δ- P需要一个“锁定机制”,其中缔合和解离都需要DNA双链体的较大长度增加。相反,对力依赖性结合动力学的测量表明,Δ,Δ- B需要较大的DNA长度增加以进行缔合,但不增加长度以使其与DNA分离。这与常规嵌入剂形成鲜明对比,常规嵌入剂几乎不需要DNA长度变化即可缔合,但解离必须发生较大的DNA长度变化。该结果说明了与传统插层相比根本不同的穿线插层机理,并将为基于DNA穿线插层的合理设计治疗药物铺平道路。
更新日期:2017-12-15
中文翻译:

重塑能量格局改变了DNA穿插的机理和结合动力学
通过穿线插入结合DNA的分子显示出高的结合亲和力和缓慢的解离动力学,这是开发抗癌药物的理想特性。为此,至关重要的是要确定导致最佳DNA相互作用的穿线嵌入剂的特定分子特征。使用单分子技术,我们可以量化小金属-有机钌穿线嵌入剂(Δ,Δ- B)的结合,并比较其与具有明显更大穿线部分(Δ,Δ- P)的相似分子的结合特性。两个分子的结合亲和力相同,而结合动力学的比较表明,Δ,Δ- B的动力学明显更快。。然而,动力学仍然比常规嵌入剂观察到的慢得多。两种穿线嵌入剂的比较表明,结合亲和力由嵌入部分独立调节,而结合动力学由穿线部分调节。为了穿入DNA,Δ,Δ- P需要一个“锁定机制”,其中缔合和解离都需要DNA双链体的较大长度增加。相反,对力依赖性结合动力学的测量表明,Δ,Δ- B需要较大的DNA长度增加以进行缔合,但不增加长度以使其与DNA分离。这与常规嵌入剂形成鲜明对比,常规嵌入剂几乎不需要DNA长度变化即可缔合,但解离必须发生较大的DNA长度变化。该结果说明了与传统插层相比根本不同的穿线插层机理,并将为基于DNA穿线插层的合理设计治疗药物铺平道路。



























 京公网安备 11010802027423号
京公网安备 11010802027423号