Molecular Cell ( IF 14.5 ) Pub Date : 2017-12-14 , DOI: 10.1016/j.molcel.2017.11.016 Kobi Simpson-Lavy , Tianchang Xu , Mark Johnston , Martin Kupiec
|
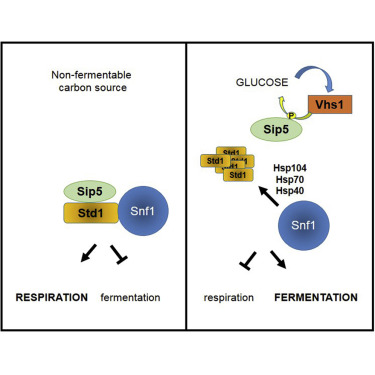
The ability to respond to available nutrients is critical for all living cells. The AMP-activated protein kinase (SNF1 in yeast) is a central regulator of metabolism that is activated when energy is depleted. We found that SNF1 activity in the nucleus is regulated by controlled relocalization of the SNF1 activator Std1 into puncta. This process is regulated by glucose through the activity of the previously uncharacterized protein kinase Vhs1 and its substrate Sip5, a protein of hitherto unknown function. Phosphorylation of Sip5 prevents its association with Std1 and triggers Std1 accretion. Reversible Std1 puncta formation occurs under non-stressful, ambient conditions, creating non-amyloid inclusion bodies at the nuclear-vacuolar junction, and it utilizes cellular chaperones similarly to the aggregation of toxic or misfolded proteins such as those associated with Parkinson’s, Alzheimer’s, and CJD diseases. Our results reveal a controlled, non-pathological, physiological role of protein aggregation in the regulation of a major metabolic cellular pathway.
中文翻译:

Snf1 / AMPK激酶的Std1激活剂通过调节蛋白聚集来控制酵母中的葡萄糖反应。
对可用养分作出反应的能力对于所有活细胞都是至关重要的。AMP激活的蛋白激酶(酵母中的SNF1)是新陈代谢的中央调节器,能量耗尽后就会激活。我们发现,SNF1激活子Std1受控地重新定位到点中,从而调节了细胞核中SNF1的活性。葡萄糖通过先前未表征的蛋白激酶Vhs1及其底物Sip5(迄今未知功能的蛋白)的活性来调节葡萄糖。Sip5的磷酸化可防止其与Std1缔合并触发Std1积聚。可逆的Std1点形成在非压力的环境条件下发生,在核-真空连接处形成非淀粉样的包涵体,而且它利用细胞伴侣的方式类似于聚集有毒或折叠错误的蛋白质,例如与帕金森氏症,阿尔茨海默氏病和克雅氏病相关的蛋白质。我们的结果揭示了蛋白质聚集在主要代谢细胞途径调控中的受控,非病理生理作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号