当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective synthesis of novel pyrano[3,2-c]chromene derivatives as AChE inhibitors via an organocatalytic domino reaction†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-12-14 00:00:00 , DOI: 10.1039/c7ob02794j Jie Zheng 1, 2, 3, 4, 5 , Ming He 1, 2, 3, 4, 5 , Baohua Xie 1, 2, 3, 4, 5 , Lu Yang 1, 2, 3, 4, 5 , Zhiye Hu 1, 2, 3, 4, 5 , Hai-Bing Zhou 1, 2, 3, 4, 5 , Chune Dong 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-12-14 00:00:00 , DOI: 10.1039/c7ob02794j Jie Zheng 1, 2, 3, 4, 5 , Ming He 1, 2, 3, 4, 5 , Baohua Xie 1, 2, 3, 4, 5 , Lu Yang 1, 2, 3, 4, 5 , Zhiye Hu 1, 2, 3, 4, 5 , Hai-Bing Zhou 1, 2, 3, 4, 5 , Chune Dong 1, 2, 3, 4, 5
Affiliation
|
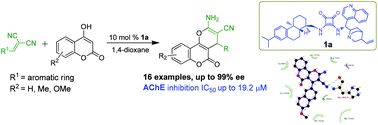
A series of optically active pyrano[3,2-c]chromenes have been synthesized through an asymmetric domino reaction of 4-hydroxy-2H-chromen-2-ones with malononitriles. The targeted molecules were obtained in excellent yields and enantioselectivities (up to 94% yield, 99% ee). The AChE inhibitory activity studies revealed that compounds 4n (IC50 = 21.3 μM) and 4p (IC50 = 19.2 μM) displayed potent acetylcholinesterase inhibition. In most cases, the S-enantiomers were superior to the corresponding R-enantiomers. Moreover, molecular modelling provides a practical method for understanding the enantioselective discrimination of AChE with these kinds of compounds.
中文翻译:

通过有机催化多米诺反应对作为AChE抑制剂的 吡喃并[3,2- c ]色烯衍生物进行对映选择性合成†
通过4-羟基-2 H-苯甲基-2-酮与丙二腈的不对称多米诺反应,合成了一系列旋光的吡喃并[3,2- c ]色烯。以优异的收率和对映选择性(高达94%的收率,99%ee)获得了目标分子。AChE抑制活性研究表明,化合物4n(IC 50 = 21.3μM)和4p(IC 50 = 19.2μM)显示出有效的乙酰胆碱酯酶抑制作用。在大多数情况下,S-对映异构体优于相应的R-对映体。此外,分子建模为理解这类化合物对AChE的对映选择性提供了一种实用的方法。
更新日期:2017-12-14
中文翻译:

通过有机催化多米诺反应对作为AChE抑制剂的 吡喃并[3,2- c ]色烯衍生物进行对映选择性合成†
通过4-羟基-2 H-苯甲基-2-酮与丙二腈的不对称多米诺反应,合成了一系列旋光的吡喃并[3,2- c ]色烯。以优异的收率和对映选择性(高达94%的收率,99%ee)获得了目标分子。AChE抑制活性研究表明,化合物4n(IC 50 = 21.3μM)和4p(IC 50 = 19.2μM)显示出有效的乙酰胆碱酯酶抑制作用。在大多数情况下,S-对映异构体优于相应的R-对映体。此外,分子建模为理解这类化合物对AChE的对映选择性提供了一种实用的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号