Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Distinct interface behaviors of Ni(ii) on graphene oxide and oxidized carbon nanotubes triggered by different topological aggregations†
Nanoscale ( IF 5.8 ) Pub Date : 2017-12-14 00:00:00 , DOI: 10.1039/c7nr07966d Jianjun Liang 1, 2, 3, 4, 5 , Ping Li 1, 2, 3, 4, 5 , Xiaolan Zhao 1, 2, 3, 4, 5 , Ziyi Liu 4, 6, 7, 8, 9 , Qiaohui Fan 1, 2, 3, 4, 5 , Zhan Li 4, 5, 10, 11, 12 , Jiaxing Li 4, 13, 14, 15 , Dongqi Wang 4, 6, 7, 8, 9
Nanoscale ( IF 5.8 ) Pub Date : 2017-12-14 00:00:00 , DOI: 10.1039/c7nr07966d Jianjun Liang 1, 2, 3, 4, 5 , Ping Li 1, 2, 3, 4, 5 , Xiaolan Zhao 1, 2, 3, 4, 5 , Ziyi Liu 4, 6, 7, 8, 9 , Qiaohui Fan 1, 2, 3, 4, 5 , Zhan Li 4, 5, 10, 11, 12 , Jiaxing Li 4, 13, 14, 15 , Dongqi Wang 4, 6, 7, 8, 9
Affiliation
|
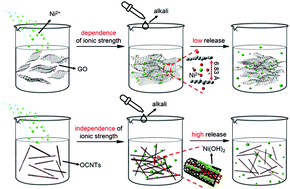
Although carbon nanotubes can be described as a seamlessly curled graphene nanosheet, two-dimensional graphene oxide (GO) and one-dimensional oxidized carbon nanotubes (OCNTs) have different fates and environmental risks, such as deposition, degradation and cytotoxicity. In particular, coexisting heavy metal ions (HMs) trigger distinct morphological transformations of both of these carbon derivatives. In addition, these morphological transformations can change the interface behaviors of HMs on both of these materials. In this study, the differences in the morphological changes of GO and OCNTs and the interface behaviors of Ni(II) were explored via the intrinsically microscopic structural changes of both of these typical carbon materials. Batch experiments revealed that Ni(II) sorption on GO drastically decreased with increasing ionic strength, while it was almost independent of ionic strength on the OCNTs. This phenomenon is attributed to the aggregation and wrinkling of GO sheets at higher Na+ concentrations, resulting in a decrease in the GO surface area and number of sorption sites. Meanwhile, the intertwining aggregations of OCNTs still ensured that the sorption sites were naked. For the first time, Ni2+ ions were observed to persist as inner-sphere complexes on GO even under alkaline conditions, where the Ni(OH)2(s) phase was determined on the OCNTs. This could be attributed to the fact that the fast aggregation of GO, which fixed Ni2+ ions into the interlayers, inhibited the nucleation of Ni(OH)2. Stable layered structures of GO aggregations were difficult to exfoliate, leading to a decreased release of Ni(II) from GO with increasing Ni(II) loading. For the OCNTs, naked Ni2+ ions could be easily and effectively released. These findings are critical to assess the mobility, transformation and cytotoxicity of nanomaterials and HMs in aquatic environments.
中文翻译:

不同拓扑聚集引发 的Ni(ii)在氧化石墨烯和氧化的碳纳米管上的明显界面行为†
尽管碳纳米管可以描述为无缝卷曲的石墨烯纳米片,但是二维氧化石墨烯(GO)和一维氧化碳纳米管(OCNT)具有不同的命运和环境风险,例如沉积,降解和细胞毒性。特别是,共存的重金属离子(HMs)触发了这两种碳衍生物的独特形态转变。另外,这些形态转变可以改变HMs在这两种材料上的界面行为。在这项研究中,通过这两种典型碳材料的固有微观结构变化,探讨了GO和OCNT的形态变化以及Ni(II)的界面行为的差异。批处理实验表明,Ni(II)随着离子强度的增加,GO上的吸附量急剧下降,而与OCNT上的离子强度几乎无关。这种现象归因于在较高的Na +浓度下GO片的聚集和起皱,导致GO表面积和吸附位点数量减少。同时,OCNT的相互缠结的聚集仍然确保了吸附位是裸露的。首次观察到甚至在碱性条件下,Ni 2+离子仍作为内球络合物在GO上持久存在,在碱性条件下,在OCNT上确定了Ni(OH)2(s)相。这可能归因于GO的快速聚集,它固定了Ni 2+离子进入中间层,抑制了Ni(OH)2的成核。GO团聚体的稳定分层结构难以剥落,导致Ni(II)负载增加,从而降低了GO中Ni(II)的释放。对于OCNT,可以轻松有效地释放裸露的Ni 2+离子。这些发现对于评估纳米材料和HM在水生环境中的迁移,转化和细胞毒性至关重要。
更新日期:2017-12-14
中文翻译:

不同拓扑聚集引发 的Ni(ii)在氧化石墨烯和氧化的碳纳米管上的明显界面行为†
尽管碳纳米管可以描述为无缝卷曲的石墨烯纳米片,但是二维氧化石墨烯(GO)和一维氧化碳纳米管(OCNT)具有不同的命运和环境风险,例如沉积,降解和细胞毒性。特别是,共存的重金属离子(HMs)触发了这两种碳衍生物的独特形态转变。另外,这些形态转变可以改变HMs在这两种材料上的界面行为。在这项研究中,通过这两种典型碳材料的固有微观结构变化,探讨了GO和OCNT的形态变化以及Ni(II)的界面行为的差异。批处理实验表明,Ni(II)随着离子强度的增加,GO上的吸附量急剧下降,而与OCNT上的离子强度几乎无关。这种现象归因于在较高的Na +浓度下GO片的聚集和起皱,导致GO表面积和吸附位点数量减少。同时,OCNT的相互缠结的聚集仍然确保了吸附位是裸露的。首次观察到甚至在碱性条件下,Ni 2+离子仍作为内球络合物在GO上持久存在,在碱性条件下,在OCNT上确定了Ni(OH)2(s)相。这可能归因于GO的快速聚集,它固定了Ni 2+离子进入中间层,抑制了Ni(OH)2的成核。GO团聚体的稳定分层结构难以剥落,导致Ni(II)负载增加,从而降低了GO中Ni(II)的释放。对于OCNT,可以轻松有效地释放裸露的Ni 2+离子。这些发现对于评估纳米材料和HM在水生环境中的迁移,转化和细胞毒性至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号