当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A structurally-characterized peroxomanganese(iv) porphyrin from reversible O2 binding within a metal–organic framework†
Chemical Science ( IF 7.6 ) Pub Date : 2017-12-14 00:00:00 , DOI: 10.1039/c7sc03739b Audrey T Gallagher 1 , Jung Yoon Lee 1 , Venkatesan Kathiresan 1 , John S Anderson 1 , Brian M Hoffman 1 , T David Harris 1
Chemical Science ( IF 7.6 ) Pub Date : 2017-12-14 00:00:00 , DOI: 10.1039/c7sc03739b Audrey T Gallagher 1 , Jung Yoon Lee 1 , Venkatesan Kathiresan 1 , John S Anderson 1 , Brian M Hoffman 1 , T David Harris 1
Affiliation
|
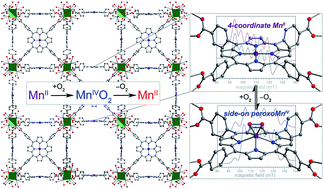
The role of peroxometal species as reactive intermediates in myriad biological processes has motivated the synthesis and study of analogous molecular model complexes. Peroxomanganese(IV) porphyrin complexes are of particular interest, owing to their potential ability to form from reversible O2 binding, yet have been exceedingly difficult to isolate and characterize in molecular form. Alternatively, immobilization of metalloporphyrin sites within a metal–organic framework (MOF) can enable the study of interactions between low-coordinate metal centers and gaseous substrates, without interference from bimolecular reactions and axial ligation by solvent molecules. Here, we employ this approach to isolate the first rigorously four-coordinate manganese(II) porphyrin complex and examine its reactivity with O2 using infrared spectroscopy, single-crystal X-ray diffraction, EPR spectroscopy, and O2 adsorption analysis. X-ray diffraction experiments reveal for the first time a peroxomanganese(IV) porphyrin species, which exhibits a side-on, η2 binding mode. Infrared and EPR spectroscopic data confirm the formulation of a peroxomanganese(IV) electronic structure, and show that O2 binding is reversible at ambient temperature, in contrast to what has been observed in molecular form. Finally, O2 gas adsorption measurements are employed to quantify the enthalpy of O2 binding as hads = −49.6(8) kJ mol−1. This enthalpy is considerably higher than in the corresponding Fe- and Co-based MOFs, and is found to increase with increasing reductive capacity of the MII/III redox couple.
中文翻译:

一种具有结构特征的过氧锰(iv)卟啉,来自金属有机框架内可逆的 O2 结合†
过氧金属物质作为多种生物过程中的反应中间体的作用促进了类似分子模型复合物的合成和研究。过氧化锰( IV )卟啉复合物由于其通过可逆O 2结合形成的潜在能力而受到特别关注,但以分子形式分离和表征却极其困难。或者,将金属卟啉位点固定在金属有机框架(MOF)内可以研究低配位金属中心和气态底物之间的相互作用,而不会受到双分子反应和溶剂分子轴向连接的干扰。在这里,我们采用这种方法分离出第一个严格的四配位锰( II )卟啉配合物,并使用红外光谱、单晶X射线衍射、EPR光谱和O 2吸附分析检查其与O 2的反应性。 X射线衍射实验首次揭示了过氧锰( IV )卟啉物种,它表现出侧向、η 2结合模式。红外和 EPR 光谱数据证实了过氧锰 ( IV ) 电子结构的形成,并表明 O 2结合在环境温度下是可逆的,这与在分子形式中观察到的情况相反。 最后,采用O 2气体吸附测量来量化O 2结合的焓,即h ads = -49.6(8) kJ mol -1 。该焓明显高于相应的铁基和钴基 MOF,并且发现随着 M II/III氧化还原电对还原能力的增加而增加。
更新日期:2017-12-14
中文翻译:

一种具有结构特征的过氧锰(iv)卟啉,来自金属有机框架内可逆的 O2 结合†
过氧金属物质作为多种生物过程中的反应中间体的作用促进了类似分子模型复合物的合成和研究。过氧化锰( IV )卟啉复合物由于其通过可逆O 2结合形成的潜在能力而受到特别关注,但以分子形式分离和表征却极其困难。或者,将金属卟啉位点固定在金属有机框架(MOF)内可以研究低配位金属中心和气态底物之间的相互作用,而不会受到双分子反应和溶剂分子轴向连接的干扰。在这里,我们采用这种方法分离出第一个严格的四配位锰( II )卟啉配合物,并使用红外光谱、单晶X射线衍射、EPR光谱和O 2吸附分析检查其与O 2的反应性。 X射线衍射实验首次揭示了过氧锰( IV )卟啉物种,它表现出侧向、η 2结合模式。红外和 EPR 光谱数据证实了过氧锰 ( IV ) 电子结构的形成,并表明 O 2结合在环境温度下是可逆的,这与在分子形式中观察到的情况相反。 最后,采用O 2气体吸附测量来量化O 2结合的焓,即h ads = -49.6(8) kJ mol -1 。该焓明显高于相应的铁基和钴基 MOF,并且发现随着 M II/III氧化还原电对还原能力的增加而增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号