当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and kinetic resolution of substituted tetrahydroquinolines by lithiation then electrophilic quench†
Chemical Science ( IF 8.4 ) Pub Date : 2017-12-14 00:00:00 , DOI: 10.1039/c7sc04435f Nicholas Carter 1 , Xiabing Li 1 , Lewis Reavey 1 , Anthony J H M Meijer 1 , Iain Coldham 1
Chemical Science ( IF 8.4 ) Pub Date : 2017-12-14 00:00:00 , DOI: 10.1039/c7sc04435f Nicholas Carter 1 , Xiabing Li 1 , Lewis Reavey 1 , Anthony J H M Meijer 1 , Iain Coldham 1
Affiliation
|
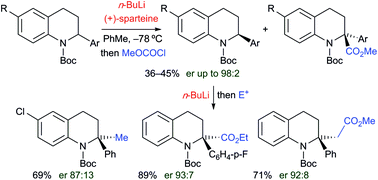
Treatment of N-Boc-2-aryl-1,2,3,4-tetrahydroquinolines with n-butyllithium in THF at −78 °C resulted in efficient lithiation at the 2-position and the organolithiums were trapped with a variety of electrophiles to give substituted products. Variable temperature NMR spectroscopy gave kinetic data that showed that the rate of tert-butoxycarbonyl (Boc) rotation was fast (ΔG‡ ≈ 45 kJ mol−1 at −78 °C) and in situ ReactIR spectroscopy showed fast lithiation at −78 °C. By carrying out the lithiation in the presence of the chiral ligand sparteine, kinetic resolutions with very high levels of enantioselectivity were achieved. The resulting enantioenriched N-Boc-2-aryltetrahydroquinolines were converted to 2,2-disubstituted products without significant loss in enantiopurity. Most electrophiles add at the 2-position and the chemistry provides a way to access tetrahydroquinolines that are fully substituted alpha to the nitrogen atom. Notably, either enantiomer of the 2,2-disubstituted tetrahydroquinolines can be obtained with high selectivity from the same enantiomer of the chiral ligand. Unusually, when methyl cyanoformate was used as the electrophile, substitution occurred in the ortho position of the aryl ring attached at C-2. This change in regioselectivity on changing the electrophile was probed by deuterium isotope studies and by DFT calculations which suggested that the binding of the cyanoformate altered the structure of the intermediate organolithium. Secondary amine products can be prepared by removing the Boc group with acid or by inducing the Boc group to rearrange to the 2-position in the presence of triethylborane and this carbonyl N-to-C rearrangement occurs with retention of configuration from the intermediate enantiomerically enriched organolithium species.
中文翻译:

锂化然后亲电猝灭取代四氢喹啉的合成和动力学拆分†
在-78 °C 下,在 THF 中用正丁基锂处理N -Boc -2-aryl-1,2,3,4-四氢喹啉导致 2 位有效锂化,有机锂被各种亲电试剂捕获给出替代产品。变温 NMR 光谱给出的动力学数据表明,叔丁氧羰基 (Boc) 的旋转速率很快(Δ G ‡ ≈ 45 kJ mol -1 at -78 °C),原位ReactIR 光谱显示在 -78 ° 下快速锂化C。通过在手性配体sparteine 存在下进行锂化,实现了具有非常高水平的对映选择性的动力学拆分。所得对映体富集N-Boc-2-芳基四氢喹啉被转化为 2,2-二取代产物,而对映体纯度没有显着损失。大多数亲电试剂在 2 位添加,化学提供了一种获得四氢喹啉的方法,四氢喹啉在氮原子的 α 位上完全取代。值得注意的是,2,2-二取代四氢喹啉的任一对映异构体都可以从手性配体的相同对映异构体中以高选择性获得。不同寻常的是,当氰基甲酸甲酯用作亲电子试剂时,取代发生在连接在 C-2 上的芳基环的邻位。通过氘同位素研究和 DFT 计算探测了改变亲电试剂时区域选择性的这种变化,这表明氰基甲酸酯的结合改变了中间体有机锂的结构。
更新日期:2017-12-14
中文翻译:

锂化然后亲电猝灭取代四氢喹啉的合成和动力学拆分†
在-78 °C 下,在 THF 中用正丁基锂处理N -Boc -2-aryl-1,2,3,4-四氢喹啉导致 2 位有效锂化,有机锂被各种亲电试剂捕获给出替代产品。变温 NMR 光谱给出的动力学数据表明,叔丁氧羰基 (Boc) 的旋转速率很快(Δ G ‡ ≈ 45 kJ mol -1 at -78 °C),原位ReactIR 光谱显示在 -78 ° 下快速锂化C。通过在手性配体sparteine 存在下进行锂化,实现了具有非常高水平的对映选择性的动力学拆分。所得对映体富集N-Boc-2-芳基四氢喹啉被转化为 2,2-二取代产物,而对映体纯度没有显着损失。大多数亲电试剂在 2 位添加,化学提供了一种获得四氢喹啉的方法,四氢喹啉在氮原子的 α 位上完全取代。值得注意的是,2,2-二取代四氢喹啉的任一对映异构体都可以从手性配体的相同对映异构体中以高选择性获得。不同寻常的是,当氰基甲酸甲酯用作亲电子试剂时,取代发生在连接在 C-2 上的芳基环的邻位。通过氘同位素研究和 DFT 计算探测了改变亲电试剂时区域选择性的这种变化,这表明氰基甲酸酯的结合改变了中间体有机锂的结构。



























 京公网安备 11010802027423号
京公网安备 11010802027423号