Chem ( IF 19.1 ) Pub Date : 2017-12-14 , DOI: 10.1016/j.chempr.2017.10.001 Shan Tang , Yichang Liu , Aiwen Lei
|
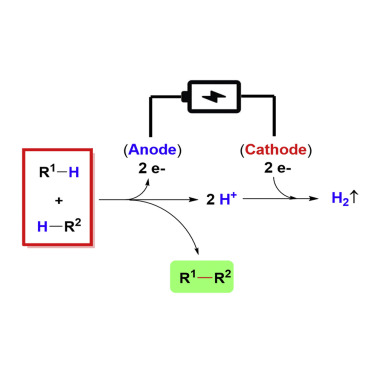
Oxidative R1–H/R2–H cross-coupling represents an ideal way for the construction of new chemical bonds. However, the bond formation with loss of H2 is typically thermodynamically unfavorable and thus usually requires an external driving force, namely, an appropriate sacrificial oxidant. Recent advances have revealed that oxidative R1–H/R2–H cross-coupling with hydrogen gas evolution can be achieved through electrochemical anodic oxidation and concomitant cathodic proton reduction. Electrochemistry provides new opportunities for the construction of carbon-carbon and carbon-heteroatom bonds in an environmentally friendly manner. This review article gives an overview of the recent developments in this emerging field.
中文翻译:

电化学氧化与氢释放的交叉耦合:绿色和可持续的债券形成方式。
R 1 -H / R 2 -H的氧化交叉偶联是构建新化学键的理想方法。然而,具有H 2损失的键形成通常在热力学上是不利的,因此通常需要外部驱动力,即适当的牺牲氧化剂。最近的进展表明,氧化R 1 –H / R 2通过电化学阳极氧化和伴随的阴极质子还原,可以实现-H与氢气逸出的交叉偶联。电化学为以环境友好的方式构建碳-碳和碳-杂原子键提供了新的机会。这篇综述文章概述了这个新兴领域的最新发展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号