Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2017-12-14 , DOI: 10.1016/j.bmc.2017.11.017 Rui-Yang Zhang , Parashar Thapa , Michael J. Espiritu , Vinay Menon , Jon-Paul Bingham
|
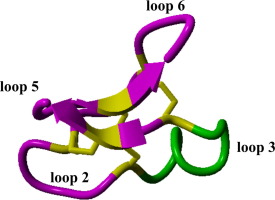
Cyclic peptides and cyclotides are becoming common identities within the present efforts seen in peptide engineering – as we seek approaches to achieve potent biological activity, pharmacological selectivity, structurally stability and oral bioavailability. Yet this unique family of peptides has faced uncommon hurdles in their discovery, synthesis and bioengineering, retaining to characteristics that truly deviate these from their linear counterparts. In this mini-review we take a board spectrum approach to introduce this novel family of biomolecules and the troubles that they face in their sequence and disulfide connectivity assignment, together highlighting the present combined strategies involved in cyclic peptide/cyclotide synthesis and modification. These efforts have circumvented otherwise impossible hurdles in their manipulation and production that are only now advancing cyclic peptides/cyclotides as research probes and future pharmaceutical templates.
中文翻译:

从自然到创造:四处走动,肽环化的艺术
在肽工程领域,在我们目前的努力中,环肽和环氧化物正成为常见的身份-我们正在寻求实现有效的生物活性,药理选择性,结构稳定性和口服生物利用度的方法。然而,这种独特的肽家族在其发现,合成和生物工程方面面临着罕见的障碍,保留了真正使它们与线性对应物偏离的特性。在本微型综述中,我们将采用板级光谱方法来介绍这一新颖的生物分子家族,以及它们在序列和二硫键连接性分配方面所面临的麻烦,共同强调当前涉及环肽/环氧化物合成和修饰的联合策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号