当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
OFF–ON Fluorescence Sensing of Fluoride by Donor–Antimony(V) Lewis Acids
Organometallics ( IF 2.5 ) Pub Date : 2017-12-14 00:00:00 , DOI: 10.1021/acs.organomet.7b00759 Ajay Kumar 1 , Mengxi Yang 2 , Minji Kim 1 , François P. Gabbaï 2 , Min Hyung Lee 1
Organometallics ( IF 2.5 ) Pub Date : 2017-12-14 00:00:00 , DOI: 10.1021/acs.organomet.7b00759 Ajay Kumar 1 , Mengxi Yang 2 , Minji Kim 1 , François P. Gabbaï 2 , Min Hyung Lee 1
Affiliation
|
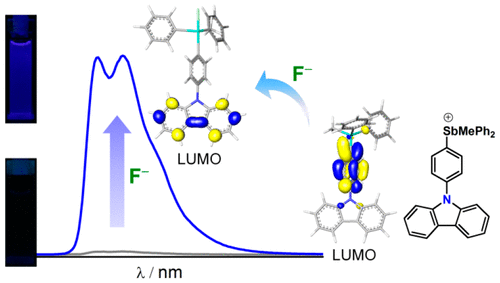
A series of triarylmethylstibonium Lewis acids of general formula [Ph2MeSb-(p-(C6H4))-FLUO]+ bearing a peripheral electron-rich fluorophore (FLUO = 10H-phenoxazine ([3a]+), diphenylamine ([3b]+), and 9H-carbazole ([3c]+)) have been synthesized and investigated for the fluorescence turn-on sensing of fluoride anions. Treatment of the stibonium cations with fluoride anions leads to the corresponding fluorostiboranes (3a-F–3c-F). While the stibonium cations are almost nonemissive, the fluorostiboranes display fluorophore-centered emissions arising from the corresponding π–π* excited state. The carbazole-containing derivative [3c]+ exhibits the most intense fluorescence turn-on response. It also displays a high binding constant (K > 107 M–1) in MeCN and shows compatibility with protic media such as MeOH (K = 950(±50) M–1). Computational studies aimed at identifying the origin of the turn-on response show that the excited state of the stibonium cations is best described as charge transfer in nature with the π system of the fluorophore acting as the donor and the π*−σ* system of the stibonium unit acting as the acceptor. This π(FLUO)−π*/σ*(Ph2MeSb-(p-(C6H4))) excited state is nonemissive, making these cations dark in the absence of fluoride anions. Conversion to the fluorostiboranes occurs via donation of a fluoride lone pair into the antimony-centered σ*. Formation of this Sb–F bond modifies the electronic structure of the platform and restores the emissive π–π* excited state of the fluorophore, thus accounting for the observed OFF–ON fluorescence response.
中文翻译:

供体-锑(V)路易斯酸对氟的OFF-ON荧光传感
一系列通式为[Ph 2 MeSb-(p-(C 6 H 4))-FLUO] +的三芳基甲基sti路易斯酸,带有外围富电子荧光团(FLUO = 10 H-吩恶嗪([ 3a ] +),二苯胺([ 3b ] +)和9 H-咔唑([ 3c ] +)已被合成并研究了氟阴离子的荧光导通。用氟化物阴离子处理sti阳离子产生相应的氟bor硼烷(3a -F– 3c-F)。尽管stibonium阳离子几乎是不发光的,但氟硼硼烷显示出由相应的π–π *激发态引起的以荧光团为中心的发射。含咔唑的衍生物[ 3c ] +表现出最强的荧光开启响应。它还在MeCN中显示出高的结合常数(K > 10 7 M –1),并显示与质子介质如MeOH的相容性(K = 950(±50)M –1)。旨在确定开启响应起源的计算研究表明,最好的方式是将stibonium阳离子的激发态描述为电荷转移,其中荧光团的π系统充当供体,而π*-σ*系统充当荧光团。充当接收器的stibonium单元。该π(FLUO)−π * /σ*(Ph 2 MeSb-(p-(C 6 H 4)))激发态是不容许的,在没有氟化物阴离子的情况下使这些阳离子变暗。通过将氟化物孤对捐赠给以锑为中心的σ*,可以转化为氟乙硼烷。该Sb-F键的形成修饰了平台的电子结构,并恢复了荧光团的发射π-π*激发态,因此可以解释观察到的OFF-ON荧光响应。
更新日期:2017-12-15
中文翻译:

供体-锑(V)路易斯酸对氟的OFF-ON荧光传感
一系列通式为[Ph 2 MeSb-(p-(C 6 H 4))-FLUO] +的三芳基甲基sti路易斯酸,带有外围富电子荧光团(FLUO = 10 H-吩恶嗪([ 3a ] +),二苯胺([ 3b ] +)和9 H-咔唑([ 3c ] +)已被合成并研究了氟阴离子的荧光导通。用氟化物阴离子处理sti阳离子产生相应的氟bor硼烷(3a -F– 3c-F)。尽管stibonium阳离子几乎是不发光的,但氟硼硼烷显示出由相应的π–π *激发态引起的以荧光团为中心的发射。含咔唑的衍生物[ 3c ] +表现出最强的荧光开启响应。它还在MeCN中显示出高的结合常数(K > 10 7 M –1),并显示与质子介质如MeOH的相容性(K = 950(±50)M –1)。旨在确定开启响应起源的计算研究表明,最好的方式是将stibonium阳离子的激发态描述为电荷转移,其中荧光团的π系统充当供体,而π*-σ*系统充当荧光团。充当接收器的stibonium单元。该π(FLUO)−π * /σ*(Ph 2 MeSb-(p-(C 6 H 4)))激发态是不容许的,在没有氟化物阴离子的情况下使这些阳离子变暗。通过将氟化物孤对捐赠给以锑为中心的σ*,可以转化为氟乙硼烷。该Sb-F键的形成修饰了平台的电子结构,并恢复了荧光团的发射π-π*激发态,因此可以解释观察到的OFF-ON荧光响应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号