当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structures and Heats of Formation of Simple Alkaline Earth Metal Compounds II: Fluorides, Chlorides, Oxides, and Hydroxides for Ba, Sr, and Ra
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acs.jpca.7b09056 Monica Vasiliu 1 , J. Grant Hill 2 , Kirk A. Peterson 3 , David A. Dixon 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acs.jpca.7b09056 Monica Vasiliu 1 , J. Grant Hill 2 , Kirk A. Peterson 3 , David A. Dixon 1
Affiliation
|
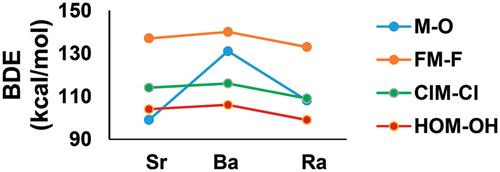
Geometry parameters, vibrational frequencies, heats of formation, bond dissociation energies, cohesive energies, and selected fluoride affinities (difluorides) are predicted for the late alkaline earth (Sr, Ba, and Ra) oxides, fluorides, chlorides, and hydroxides at the coupled cluster theory CCSD(T) level. Additional corrections (scalar relativistic and pseudopotential corrections, vibrational zero-point energies, and atomic spin–orbit effects) were included to accurately calculate the total atomization energies and heats of formation following the Feller–Peterson–Dixon methodology. The calculated values are compared to the experimental data where available. In some cases, especially for Ra compounds, there are no experimental results, or the experimental energetics and geometries are not reliable or have very large error bars. All of the Sr, Ba, and Ra difluorides, dichlorides, and dihydroxides are bent structures with the OMO bond angles decreasing going down the group. The cohesive energies of bulk Be dihalides are predicted to be quite low, while those of Ra are relatively large. The fluoride affinities show that the difluorides are moderately strong Lewis acids and that such trifluorides may form under the appropriate experimental conditions.
中文翻译:

简单的碱土金属化合物II的结构和形成热:Ba,Sr和Ra的氟化物,氯化物,氧化物和氢氧化物
预测了耦合后的碱土金属(Sr,Ba和Ra)的氧化物,氟化物,氯化物和氢氧化物的几何参数,振动频率,形成热,键解离能,内聚能和选定的氟化物亲和力(二氟化物)聚类理论CCSD(T)级。根据Feller-Peterson-Dixon方法,还包括其他校正(标量相对论和伪势校正,振动零点能量和原子自旋轨道效应),以准确计算总雾化能量和形成热。将计算出的值与可用的实验数据进行比较。在某些情况下,尤其是对于Ra化合物,没有实验结果,或者实验的能量学和几何形状不可靠,或者误差棒很大。所有的爸,爸,Ra二氟化物,二氯化物和二氢氧化物是弯曲的结构,随着OMO键角的减小而下降。预计大块Be二卤化物的内聚能非常低,而Ra的内聚能相对较大。氟化物的亲和力表明二氟化物是中等强度的路易斯酸,并且这种三氟化物可以在适当的实验条件下形成。
更新日期:2018-01-02
中文翻译:

简单的碱土金属化合物II的结构和形成热:Ba,Sr和Ra的氟化物,氯化物,氧化物和氢氧化物
预测了耦合后的碱土金属(Sr,Ba和Ra)的氧化物,氟化物,氯化物和氢氧化物的几何参数,振动频率,形成热,键解离能,内聚能和选定的氟化物亲和力(二氟化物)聚类理论CCSD(T)级。根据Feller-Peterson-Dixon方法,还包括其他校正(标量相对论和伪势校正,振动零点能量和原子自旋轨道效应),以准确计算总雾化能量和形成热。将计算出的值与可用的实验数据进行比较。在某些情况下,尤其是对于Ra化合物,没有实验结果,或者实验的能量学和几何形状不可靠,或者误差棒很大。所有的爸,爸,Ra二氟化物,二氯化物和二氢氧化物是弯曲的结构,随着OMO键角的减小而下降。预计大块Be二卤化物的内聚能非常低,而Ra的内聚能相对较大。氟化物的亲和力表明二氟化物是中等强度的路易斯酸,并且这种三氟化物可以在适当的实验条件下形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号