Journal of Catalysis ( IF 6.5 ) Pub Date : 2017-12-14 , DOI: 10.1016/j.jcat.2017.10.002 Sujeong Kim , Gun-hee Moon , Hyejin Kim , Yeongdong Mun , Peng Zhang , Jinwoo Lee , Wonyong Choi
|
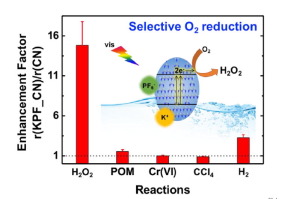
Photochemical production of H2O2 through O2 reduction has been proposed as an alternative method of solar energy storage. A carbon nitride (CN) photocatalyst was selected for this purpose. The incorporation of KPF6 into the CN structure greatly enhanced the apparent quantum yield (AQY) of H2O2 production in the UV and visible light region. The AQY of KPF6-modified CN was measured to be 35.9% and 24.3% under monochromatic irradiation at 370 and 420 nm, respectively, which are 8.3 and 26.1 times higher than for bare CN. The KPF6-enhanced activity is ascribed to several factors including (i) enhanced absorption of UV and visible light, (ii) higher charge carrier density, (iii) retarded radiative recombination of charge pairs, (iv) highly selective two-electron transfer to O2, and (v) hindered photodecomposition of in-situ generated H2O2. The markedly high selectivity of KPF6-modified CN toward the two-electron reduction of O2 (leading to H2O2) was demonstrated in comparision with other photoreductive conversions such as the reduction of polyoxometalate (POM → POM−), hexavalent chromium (CrVI → CrIII), CCl4 (dechlorination), and protons (H2 production). This study developed a simple method of efficient production of H2O2 using visible light, which could be utilized for a variety of applications that employ H2O2 as a solar fuel or a green oxidant.
中文翻译:

在可见光下选择性地转移KPF 6改性的氮化碳上的双氧来进行H 2 O 2的光催化合成
已经提出通过O 2还原的光化学生产H 2 O 2作为太阳能存储的替代方法。为此选择了氮化碳(CN)光催化剂。将KPF 6掺入CN结构中大大提高了在紫外和可见光区域中H 2 O 2产生的表观量子产率(AQY)。在单色辐射下在370和420 nm下测得的KPF 6改性CN的AQY分别为35.9%和24.3%,是裸CN的8.3倍和26.1倍。KPF 6-活性的提高归因于以下几个因素:(i)增强对紫外线和可见光的吸收,(ii)较高的载流子密度,(iii)电荷对的辐射重组减慢,(iv)高选择性的两电子转移至O 2(v)阻碍了原位生成的H 2 O 2的光分解。KPF的显着高的选择性6修饰的CN朝向的O-两个电子还原2(导致成H 2 ö 2)与其它光还原转化对比证明诸如多金属氧酸盐的还原(POM POM→ - ),六价铬(铬VI →铬III),四氯化碳4(脱氯)和质子(H 2产生)。这项研究开发了一种使用可见光有效生产H 2 O 2的简单方法,该方法可用于使用H 2 O 2作为太阳能或绿色氧化剂的各种应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号