当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Membrane Determinants Affect Fibrillation Processes of β-Sheet Charged Peptides
Biomacromolecules ( IF 5.5 ) Pub Date : 2017-12-29 00:00:00 , DOI: 10.1021/acs.biomac.7b01318 Elad Arad 1 , Ravit Malishev 1 , Hanna Rapaport 1 , Raz Jelinek 1
Biomacromolecules ( IF 5.5 ) Pub Date : 2017-12-29 00:00:00 , DOI: 10.1021/acs.biomac.7b01318 Elad Arad 1 , Ravit Malishev 1 , Hanna Rapaport 1 , Raz Jelinek 1
Affiliation
|
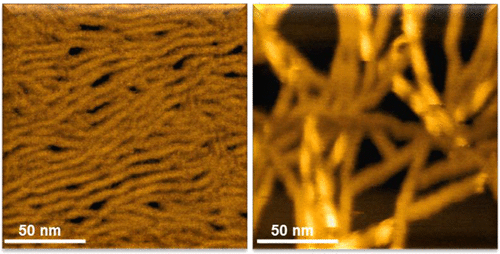
Assembly of fibrillar peptide structures is dependent both upon their intrinsic propensities toward β-structure formation, as well as on structural modulation by external molecular factors. β-sheet structures may either be designed to form useful assemblies or be the undesired consequence of protein denaturation to toxic amyloid structures in several neurodegenerative diseases. Membrane bilayers have been implicated as primary initiators and modulators of amyloid fibrillation and the reasons for this effect are yet to be elucidated. Here, we employed a set of three charged peptides having the tendency to form β-sheet fibrils, to investigate the effect of zwitterionic and negatively charged bilayer vesicles on their assembly structures. Microscopic and spectroscopic experiments revealed intimate relationship between peptide/membrane charges and fibrillation properties. Electrostatic attraction was apparent between oppositely charged peptides and vesicles; however, such interactions did not appear to significantly modulate fibril morphologies of either the net anionic peptide or the cationic one. Yet, a dramatic structural effect was observed when the nominal zwitterionic peptide underwent fibrillation in the presence of negatively charged vesicles. Assemblies of this peptide display a net positive charge, which facilitated the counterionic interactions with the vesicles. Furthermore, these interactions templated a unique twisted fiber morphology demonstrating the dramatic effect membrane-mediated interactions exert on fibril morphologies.
中文翻译:

膜决定因素影响β-Sheet荷电肽的原纤化过程
纤维状肽结构的组装既取决于它们对β-结构形成的固有倾向,也取决于外部分子因素对结构的调节。β-折叠结构可能被设计为形成有用的装配体,或者是几种神经退行性疾病中蛋白质变性为毒性淀粉样蛋白结构的不良结果。膜双层被认为是淀粉样蛋白原纤化的主要引发剂和调节剂,尚不清楚这种作用的原因。在这里,我们采用了三种具有形成β-折叠原纤维趋势的带电肽,以研究两性离子和带负电的双层囊泡对其组装结构的影响。显微镜和光谱实验表明,肽/膜电荷与原纤化性能之间存在密切的关系。带相反电荷的肽和囊泡之间有明显的静电吸引。然而,这种相互作用似乎并未显着调节净阴离子肽或阳离子肽的原纤维形态。然而,当标称的两性离子肽在带负电荷的囊泡存在下进行原纤化时,观察到了戏剧性的结构作用。该肽的组装体显示出净正电荷,其促进了与小泡的抗衡离子相互作用。此外,这些相互作用以独特的扭曲纤维形态为模板,证明了膜介导的相互作用对原纤维形态产生了显着影响。带相反电荷的肽和囊泡之间有明显的静电吸引。然而,这种相互作用似乎并未显着调节净阴离子肽或阳离子肽的原纤维形态。然而,当标称的两性离子肽在带负电荷的囊泡存在下进行原纤化时,观察到了戏剧性的结构作用。该肽的组装体显示出净正电荷,其促进了与小泡的抗衡离子相互作用。此外,这些相互作用以独特的扭曲纤维形态为模板,证明了膜介导的相互作用对原纤维形态产生了显着影响。带相反电荷的肽和囊泡之间有明显的静电吸引。然而,这种相互作用似乎并未显着调节净阴离子肽或阳离子肽的原纤维形态。然而,当标称的两性离子肽在带负电荷的囊泡存在下进行原纤化时,观察到了戏剧性的结构作用。该肽的组装体显示出净正电荷,其促进了与小泡的抗衡离子相互作用。此外,这些相互作用以独特的扭曲纤维形态为模板,证明了膜介导的相互作用对原纤维形态产生了显着影响。这种相互作用似乎并未显着调节净阴离子肽或阳离子肽的原纤维形态。然而,当标称的两性离子肽在带负电荷的囊泡存在下进行原纤化时,观察到了戏剧性的结构作用。该肽的组装体显示出净正电荷,其促进了与小泡的抗衡离子相互作用。此外,这些相互作用以独特的扭曲纤维形态为模板,证明了膜介导的相互作用对原纤维形态产生了显着影响。这种相互作用似乎并未显着调节净阴离子肽或阳离子肽的原纤维形态。然而,当标称的两性离子肽在带负电荷的囊泡存在下进行原纤化时,观察到了戏剧性的结构作用。该肽的组装体显示出净正电荷,其促进了与小泡的抗衡离子相互作用。此外,这些相互作用以独特的扭曲纤维形态为模板,证明了膜介导的相互作用对原纤维形态产生了显着影响。它促进了与小泡的抗衡离子相互作用。此外,这些相互作用以独特的扭曲纤维形态为模板,证明了膜介导的相互作用对原纤维形态产生了显着影响。它促进了与小泡的抗衡离子相互作用。此外,这些相互作用以独特的扭曲纤维形态为模板,证明了膜介导的相互作用对原纤维形态产生了显着影响。
更新日期:2017-12-29
中文翻译:

膜决定因素影响β-Sheet荷电肽的原纤化过程
纤维状肽结构的组装既取决于它们对β-结构形成的固有倾向,也取决于外部分子因素对结构的调节。β-折叠结构可能被设计为形成有用的装配体,或者是几种神经退行性疾病中蛋白质变性为毒性淀粉样蛋白结构的不良结果。膜双层被认为是淀粉样蛋白原纤化的主要引发剂和调节剂,尚不清楚这种作用的原因。在这里,我们采用了三种具有形成β-折叠原纤维趋势的带电肽,以研究两性离子和带负电的双层囊泡对其组装结构的影响。显微镜和光谱实验表明,肽/膜电荷与原纤化性能之间存在密切的关系。带相反电荷的肽和囊泡之间有明显的静电吸引。然而,这种相互作用似乎并未显着调节净阴离子肽或阳离子肽的原纤维形态。然而,当标称的两性离子肽在带负电荷的囊泡存在下进行原纤化时,观察到了戏剧性的结构作用。该肽的组装体显示出净正电荷,其促进了与小泡的抗衡离子相互作用。此外,这些相互作用以独特的扭曲纤维形态为模板,证明了膜介导的相互作用对原纤维形态产生了显着影响。带相反电荷的肽和囊泡之间有明显的静电吸引。然而,这种相互作用似乎并未显着调节净阴离子肽或阳离子肽的原纤维形态。然而,当标称的两性离子肽在带负电荷的囊泡存在下进行原纤化时,观察到了戏剧性的结构作用。该肽的组装体显示出净正电荷,其促进了与小泡的抗衡离子相互作用。此外,这些相互作用以独特的扭曲纤维形态为模板,证明了膜介导的相互作用对原纤维形态产生了显着影响。带相反电荷的肽和囊泡之间有明显的静电吸引。然而,这种相互作用似乎并未显着调节净阴离子肽或阳离子肽的原纤维形态。然而,当标称的两性离子肽在带负电荷的囊泡存在下进行原纤化时,观察到了戏剧性的结构作用。该肽的组装体显示出净正电荷,其促进了与小泡的抗衡离子相互作用。此外,这些相互作用以独特的扭曲纤维形态为模板,证明了膜介导的相互作用对原纤维形态产生了显着影响。这种相互作用似乎并未显着调节净阴离子肽或阳离子肽的原纤维形态。然而,当标称的两性离子肽在带负电荷的囊泡存在下进行原纤化时,观察到了戏剧性的结构作用。该肽的组装体显示出净正电荷,其促进了与小泡的抗衡离子相互作用。此外,这些相互作用以独特的扭曲纤维形态为模板,证明了膜介导的相互作用对原纤维形态产生了显着影响。这种相互作用似乎并未显着调节净阴离子肽或阳离子肽的原纤维形态。然而,当标称的两性离子肽在带负电荷的囊泡存在下进行原纤化时,观察到了戏剧性的结构作用。该肽的组装体显示出净正电荷,其促进了与小泡的抗衡离子相互作用。此外,这些相互作用以独特的扭曲纤维形态为模板,证明了膜介导的相互作用对原纤维形态产生了显着影响。它促进了与小泡的抗衡离子相互作用。此外,这些相互作用以独特的扭曲纤维形态为模板,证明了膜介导的相互作用对原纤维形态产生了显着影响。它促进了与小泡的抗衡离子相互作用。此外,这些相互作用以独特的扭曲纤维形态为模板,证明了膜介导的相互作用对原纤维形态产生了显着影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号