当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Variation in LOV Photoreceptor Activation Dynamics Probed by Time-Resolved Infrared Spectroscopy
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-04 00:00:00 , DOI: 10.1021/acs.biochem.7b01040 James N Iuliano 1 , Agnieszka A Gil 1 , Sergey P Laptenok 2 , Christopher R Hall 2 , Jinnette Tolentino Collado 1 , Andras Lukacs 2, 3 , Safaa A Hag Ahmed 1 , Jenna Abyad 1 , Taraneh Daryaee 1 , Gregory M Greetham 4 , Igor V Sazanovich 4 , Boris Illarionov 5 , Adelbert Bacher 6 , Markus Fischer 5 , Michael Towrie 4 , Jarrod B French 1 , Stephen R Meech 2 , Peter J Tonge 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-04 00:00:00 , DOI: 10.1021/acs.biochem.7b01040 James N Iuliano 1 , Agnieszka A Gil 1 , Sergey P Laptenok 2 , Christopher R Hall 2 , Jinnette Tolentino Collado 1 , Andras Lukacs 2, 3 , Safaa A Hag Ahmed 1 , Jenna Abyad 1 , Taraneh Daryaee 1 , Gregory M Greetham 4 , Igor V Sazanovich 4 , Boris Illarionov 5 , Adelbert Bacher 6 , Markus Fischer 5 , Michael Towrie 4 , Jarrod B French 1 , Stephen R Meech 2 , Peter J Tonge 1
Affiliation
|
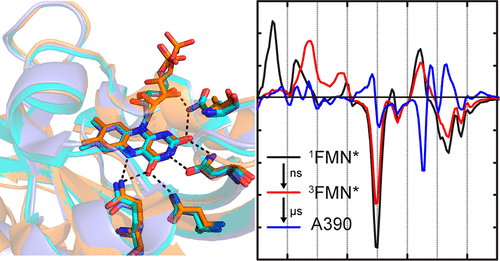
The light, oxygen, voltage (LOV) domain proteins are blue light photoreceptors that utilize a noncovalently bound flavin mononucleotide (FMN) cofactor as the chromophore. The modular nature of these proteins has led to their wide adoption in the emerging fields of optogenetics and optobiology, where the LOV domain has been fused to a variety of output domains leading to novel light-controlled applications. In this work, we extend our studies of the subpicosecond to several hundred microsecond transient infrared spectroscopy of the isolated LOV domain AsLOV2 to three full-length photoreceptors in which the LOV domain is fused to an output domain: the LOV-STAS protein, YtvA, the LOV-HTH transcription factor, EL222, and the LOV-histidine kinase, LovK. Despite differences in tertiary structure, the overall pathway leading to cysteine adduct formation from the FMN triplet state is highly conserved, although there are slight variations in rate. However, significant differences are observed in the vibrational spectra and kinetics after adduct formation, which are directly linked to the specific output function of the LOV domain. While the rate of adduct formation varies by only 3.6-fold among the proteins, the subsequent large-scale structural changes in the full-length LOV photoreceptors occur over the micro- to submillisecond time scales and vary by orders of magnitude depending on the different output function of each LOV domain.
中文翻译:

时间分辨红外光谱探测 LOV 光感受器激活动力学的变化
光、氧、电压 (LOV) 结构域蛋白是蓝光光感受器,利用非共价结合的黄素单核苷酸 (FMN) 辅因子作为发色团。这些蛋白质的模块化性质使其在光遗传学和光生物学新兴领域得到广泛采用,其中 LOV 结构域已与各种输出结构域融合,从而产生了新颖的光控制应用。在这项工作中,我们将孤立的 LOV 结构域 AsLOV2 的亚皮秒到几百微秒瞬态红外光谱的研究扩展到三个全长光感受器,其中 LOV 结构域融合到输出结构域:LOV-STAS 蛋白、YtvA、 LOV-HTH 转录因子 EL222 和 LOV-组氨酸激酶 LovK。尽管三级结构存在差异,但从 FMN 三联体状态形成半胱氨酸加合物的总体途径是高度保守的,尽管速率略有不同。然而,加合物形成后的振动光谱和动力学存在显着差异,这与 LOV 域的特定输出函数直接相关。虽然蛋白质之间加合物形成的速率仅相差 3.6 倍,但全长 LOV 光感受器随后发生的大规模结构变化发生在微到亚毫秒的时间尺度上,并且根据不同的输出而变化几个数量级每个 LOV 域的功能。
更新日期:2018-01-04
中文翻译:

时间分辨红外光谱探测 LOV 光感受器激活动力学的变化
光、氧、电压 (LOV) 结构域蛋白是蓝光光感受器,利用非共价结合的黄素单核苷酸 (FMN) 辅因子作为发色团。这些蛋白质的模块化性质使其在光遗传学和光生物学新兴领域得到广泛采用,其中 LOV 结构域已与各种输出结构域融合,从而产生了新颖的光控制应用。在这项工作中,我们将孤立的 LOV 结构域 AsLOV2 的亚皮秒到几百微秒瞬态红外光谱的研究扩展到三个全长光感受器,其中 LOV 结构域融合到输出结构域:LOV-STAS 蛋白、YtvA、 LOV-HTH 转录因子 EL222 和 LOV-组氨酸激酶 LovK。尽管三级结构存在差异,但从 FMN 三联体状态形成半胱氨酸加合物的总体途径是高度保守的,尽管速率略有不同。然而,加合物形成后的振动光谱和动力学存在显着差异,这与 LOV 域的特定输出函数直接相关。虽然蛋白质之间加合物形成的速率仅相差 3.6 倍,但全长 LOV 光感受器随后发生的大规模结构变化发生在微到亚毫秒的时间尺度上,并且根据不同的输出而变化几个数量级每个 LOV 域的功能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号