当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identifying Size Effects of Pt as Single Atoms and Nanoparticles Supported on FeOx for the Water-Gas Shift Reaction
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acscatal.7b02751 Yang Chen 1, 2 , Jian Lin 1 , Lin Li 1 , Botao Qiao 1 , Jingyue Liu 3 , Yang Su 1 , Xiaodong Wang 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acscatal.7b02751 Yang Chen 1, 2 , Jian Lin 1 , Lin Li 1 , Botao Qiao 1 , Jingyue Liu 3 , Yang Su 1 , Xiaodong Wang 1
Affiliation
|
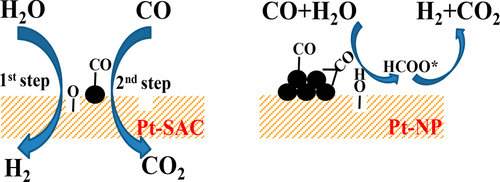
Identification of size effects at an atomic level is essential for designing high-performance metal-based catalysts. Here, the performance of a series of FeOx-supported Pt catalysts with Pt as nanoparticles (Pt-NP) or single atoms (Pt-SAC) are compared for the low-temperature water-gas shift (WGS) reaction. A variety of characterization methods such as adsorption microcalorimetry, H2-TPR, in situ DRIFTS, and transient analysis of product tests were used to demonstrate that Pt nanoparticles exhibit much higher adsorption strength of CO; the adsorbed CO reacts with the OH groups, which are generated from activated H2O, to form intermediate formates that subsequently decompose to produce CO2 and H2 simultaneously. On the other hand, Pt single atoms promote the formation of oxygen vacancies on FeOx which dissociate H2O to H2 and adsorbed O that then combines with the weakly adsorbed CO on these Pt sites to produce CO2. The activation energy for the WGS reaction decreases with the downsizing of Pt species, and Pt-SAC possesses the lowest value of 33 kJ/mol. As a result, Pt-SAC exhibits 1 order of magnitude higher specific activity in comparison to Pt-NP. With a loading of only 0.05 wt % the Pt-SAC can achieve ∼65% CO conversion at 300 °C, representing one of the most active catalysts reported so far.
中文翻译:

确定Pt作为单原子和FeO x负载的纳米粒子对水煤气变换反应的尺寸效应
识别原子级的尺寸效应对于设计高性能金属基催化剂至关重要。在此,针对低温水煤气变换(WGS)反应,比较了一系列以Pt为纳米颗粒(Pt-NP)或单原子(Pt-SAC)的FeO x负载的Pt催化剂的性能。多种表征方法,例如吸附微量量热法,H 2 -TPR,原位DRIFTS和产品测试的瞬态分析被用来证明Pt纳米颗粒表现出更高的CO吸附强度。吸附的CO与活化的H 2 O生成的OH基反应形成中间甲酸酯,随后分解生成CO 2和H 2同时。另一方面,Pt单原子促进FeO x上氧空位的形成,将H 2 O分解为H 2并吸附O,然后在这些Pt位上与弱吸附的CO结合产生CO 2。WGS反应的活化能随Pt种类的减小而降低,Pt-SAC的最低值为33 kJ / mol。结果,与Pt-NP相比,Pt-SAC显示出比活性高1个数量级。Pt-SAC的负载量仅为0.05 wt%,在300°C时可实现约65%的CO转化率,是迄今为止报道的最活跃的催化剂之一。
更新日期:2018-01-02
中文翻译:

确定Pt作为单原子和FeO x负载的纳米粒子对水煤气变换反应的尺寸效应
识别原子级的尺寸效应对于设计高性能金属基催化剂至关重要。在此,针对低温水煤气变换(WGS)反应,比较了一系列以Pt为纳米颗粒(Pt-NP)或单原子(Pt-SAC)的FeO x负载的Pt催化剂的性能。多种表征方法,例如吸附微量量热法,H 2 -TPR,原位DRIFTS和产品测试的瞬态分析被用来证明Pt纳米颗粒表现出更高的CO吸附强度。吸附的CO与活化的H 2 O生成的OH基反应形成中间甲酸酯,随后分解生成CO 2和H 2同时。另一方面,Pt单原子促进FeO x上氧空位的形成,将H 2 O分解为H 2并吸附O,然后在这些Pt位上与弱吸附的CO结合产生CO 2。WGS反应的活化能随Pt种类的减小而降低,Pt-SAC的最低值为33 kJ / mol。结果,与Pt-NP相比,Pt-SAC显示出比活性高1个数量级。Pt-SAC的负载量仅为0.05 wt%,在300°C时可实现约65%的CO转化率,是迄今为止报道的最活跃的催化剂之一。











































 京公网安备 11010802027423号
京公网安备 11010802027423号