当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Basis of ALDH1A2 Inhibition by Irreversible and Reversible Small Molecule Inhibitors
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-12-14 00:00:00 , DOI: 10.1021/acschembio.7b00685 Yan Chen 1 , Jin-Yi Zhu 1 , Kwon Ho Hong 2 , David C Mikles 1 , Gunda I Georg 2 , Alex S Goldstein 3 , John K Amory 4 , Ernst Schönbrunn 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2017-12-14 00:00:00 , DOI: 10.1021/acschembio.7b00685 Yan Chen 1 , Jin-Yi Zhu 1 , Kwon Ho Hong 2 , David C Mikles 1 , Gunda I Georg 2 , Alex S Goldstein 3 , John K Amory 4 , Ernst Schönbrunn 1
Affiliation
|
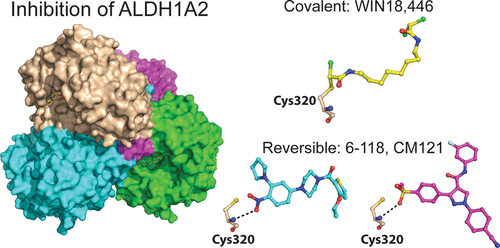
Enzymes of the ALDH1A subfamily of aldehyde dehydrogenases are crucial in regulating retinoic acid (RA) signaling and have received attention as potential drug targets. ALDH1A2 is the primary RA-synthesizing enzyme in mammalian spermatogenesis and is therefore considered a viable drug target for male contraceptive development. However, only a small number of ALDH1A2 inhibitors have been reported, and information on the structure of ALDH1A2 was limited to the NAD-liganded enzyme void of substrate or inhibitors. Herein, we describe the mechanism of action of structurally unrelated reversible and irreversible inhibitors of human ALDH1A2 using direct binding studies and X-ray crystallography. All inhibitors bind to the active sites of tetrameric ALDH1A2. Compound WIN18,446 covalently reacts with the side chain of the catalytic residue Cys320, resulting in a chiral adduct in (R) configuration. The covalent adduct directly affects the neighboring NAD molecule, which assumes a contracted conformation suboptimal for the dehydrogenase reaction. The reversible inhibitors interact predominantly through direct hydrogen bonding interactions with residues in the vicinity of Cys320 without affecting NAD. Upon interaction with inhibitors, a large flexible loop assumes regular structure, thereby shielding the active site from solvent. The precise knowledge of the binding modes provides a new framework for the rational design of novel inhibitors of ALDH1A2 with improved potency and selectivity profiles.
中文翻译:

不可逆和可逆小分子抑制剂抑制 ALDH1A2 的结构基础
乙醛脱氢酶 ALDH1A 亚家族的酶对于调节视黄酸 (RA) 信号传导至关重要,并且作为潜在的药物靶点而受到关注。 ALDH1A2 是哺乳动物精子发生中主要的 RA 合成酶,因此被认为是男性避孕药开发的可行药物靶标。然而,仅报道了少量的 ALDH1A2 抑制剂,并且有关 ALDH1A2 结构的信息仅限于缺乏底物或抑制剂的 NAD 配体酶。在此,我们使用直接结合研究和 X 射线晶体学描述了结构无关的人 ALDH1A2 可逆和不可逆抑制剂的作用机制。所有抑制剂均与四聚体 ALDH1A2 的活性位点结合。化合物 WIN18,446 与催化残基 Cys320 的侧链发生共价反应,产生 ( R ) 构型的手性加合物。共价加合物直接影响邻近的 NAD 分子,该分子呈现出对于脱氢酶反应而言不理想的收缩构象。可逆抑制剂主要通过与 Cys320 附近残基的直接氢键相互作用相互作用,而不影响 NAD。与抑制剂相互作用后,一个大的柔性环呈现规则结构,从而保护活性位点免受溶剂的影响。对结合模式的精确了解为合理设计具有改进的效力和选择性的 ALDH1A2 新型抑制剂提供了新的框架。
更新日期:2017-12-14
中文翻译:

不可逆和可逆小分子抑制剂抑制 ALDH1A2 的结构基础
乙醛脱氢酶 ALDH1A 亚家族的酶对于调节视黄酸 (RA) 信号传导至关重要,并且作为潜在的药物靶点而受到关注。 ALDH1A2 是哺乳动物精子发生中主要的 RA 合成酶,因此被认为是男性避孕药开发的可行药物靶标。然而,仅报道了少量的 ALDH1A2 抑制剂,并且有关 ALDH1A2 结构的信息仅限于缺乏底物或抑制剂的 NAD 配体酶。在此,我们使用直接结合研究和 X 射线晶体学描述了结构无关的人 ALDH1A2 可逆和不可逆抑制剂的作用机制。所有抑制剂均与四聚体 ALDH1A2 的活性位点结合。化合物 WIN18,446 与催化残基 Cys320 的侧链发生共价反应,产生 ( R ) 构型的手性加合物。共价加合物直接影响邻近的 NAD 分子,该分子呈现出对于脱氢酶反应而言不理想的收缩构象。可逆抑制剂主要通过与 Cys320 附近残基的直接氢键相互作用相互作用,而不影响 NAD。与抑制剂相互作用后,一个大的柔性环呈现规则结构,从而保护活性位点免受溶剂的影响。对结合模式的精确了解为合理设计具有改进的效力和选择性的 ALDH1A2 新型抑制剂提供了新的框架。











































 京公网安备 11010802027423号
京公网安备 11010802027423号