当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Choline-Based Amino Acid ILs–Collagen Interaction: Enunciating Its Role in Stabilization/Destabilization Phenomena
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.jpcb.7b10645 Aafiya Tarannum 1 , J. Raghava Rao 1 , N. Nishad Fathima 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.jpcb.7b10645 Aafiya Tarannum 1 , J. Raghava Rao 1 , N. Nishad Fathima 1
Affiliation
|
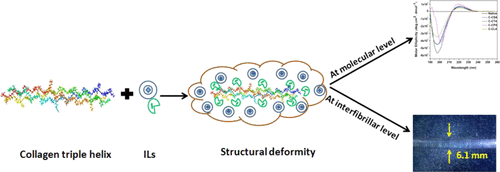
Given the potential of productive interaction between choline-based amino acid ionic liquids (CAAILs) and collagen, we investigated the role of four CAAILs, viz., choline serinate, threoninate, lysinate, and phenylalaninate, and the changes mediated by them in the structure of collagen at different hierarchical orderings, that is, at molecular and fibrillar levels. The rheological, dielectric behavior and the secondary structural changes signify the alteration in the triple helical structure of collagen at higher concentrations of CAAILs. A marginal swelling and slight decrease in the thermal stability of rat tail tendon collagen fibers were observed for choline serinate and threoninate, albeit distortions in banding patterns were noticed for choline lysinate and phenylalaninate, suggesting chaotropicity of the ions at the fibrillar level. This signifies the changes in the hydrogen-bonding environment of collagen with increasing concentrations of CAAILs, which could be due to competitive hydrogen bonding between the carbonyl group of amino acid ionic liquids and the hydroxyl groups of collagen.
中文翻译:

基于胆碱的氨基酸ILs-胶原相互作用:阐明其在稳定/去稳定现象中的作用
鉴于胆碱基氨基酸离子液体(CAAIL)和胶原蛋白之间可能产生生产性相互作用,我们研究了四种CAAILs(胆碱丝氨酸,苏氨酸,赖氨酸和苯丙氨酸)的作用,以及它们在结构中介导的变化胶原蛋白以不同的层次顺序排列,即处于分子和原纤维水平。流变,介电行为和二级结构变化表明,在较高浓度的CAAILs下,胶原蛋白的三螺旋结构发生了变化。对于胆碱丝氨酸和苏氨酸,观察到大鼠尾部腱胶原纤维的边缘肿胀和热稳定性略有降低,尽管注意到胆碱赖氨酸和苯丙氨酸盐的束带模式发生了扭曲,这表明离子在纤维状水平上具有离液性。
更新日期:2018-01-10
中文翻译:

基于胆碱的氨基酸ILs-胶原相互作用:阐明其在稳定/去稳定现象中的作用
鉴于胆碱基氨基酸离子液体(CAAIL)和胶原蛋白之间可能产生生产性相互作用,我们研究了四种CAAILs(胆碱丝氨酸,苏氨酸,赖氨酸和苯丙氨酸)的作用,以及它们在结构中介导的变化胶原蛋白以不同的层次顺序排列,即处于分子和原纤维水平。流变,介电行为和二级结构变化表明,在较高浓度的CAAILs下,胶原蛋白的三螺旋结构发生了变化。对于胆碱丝氨酸和苏氨酸,观察到大鼠尾部腱胶原纤维的边缘肿胀和热稳定性略有降低,尽管注意到胆碱赖氨酸和苯丙氨酸盐的束带模式发生了扭曲,这表明离子在纤维状水平上具有离液性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号