当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Defining Metabolic and Nonmetabolic Regulation of Histone Acetylation by NSAID Chemotypes.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-12-29 , DOI: 10.1021/acs.molpharmaceut.7b00943 Jonathan H Shrimp 1 , Julie M Garlick 1 , Tugsan Tezil 2 , Alexander W Sorum 1 , Andrew J Worth 3 , Ian A Blair 3 , Eric Verdin 2 , Nathaniel W Snyder 4 , Jordan L Meier 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-12-29 , DOI: 10.1021/acs.molpharmaceut.7b00943 Jonathan H Shrimp 1 , Julie M Garlick 1 , Tugsan Tezil 2 , Alexander W Sorum 1 , Andrew J Worth 3 , Ian A Blair 3 , Eric Verdin 2 , Nathaniel W Snyder 4 , Jordan L Meier 1
Affiliation
|
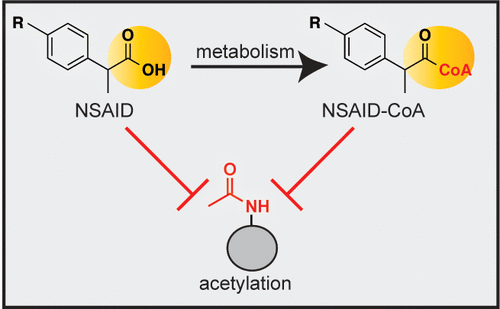
Nonsteroidal anti-inflammatory drugs (NSAIDs) are well-known for their effects on inflammatory gene expression. Although NSAIDs are known to impact multiple cellular signaling mechanisms, a recent finding is that the NSAID salicylate can disrupt histone acetylation, in part through direct inhibition of the lysine acetyltransferase (KAT) p300/CBP. While salicylate is a relatively weak KAT inhibitor, its CoA-linked metabolite is more potent; however, the ability of NSAID metabolites to inhibit KAT enzymes biochemically and in cells remains relatively unexplored. Here we define the role of metabolic and nonmetabolic mechanisms in inhibition of KAT activity by NSAID chemotypes. First, we screen a small panel of NSAIDs for biochemical inhibition of the prototypical KAT p300, leading to the finding that many carboxylate-containing NSAIDs, including ibuprofen, are able to function as weak inhibitors. Assessing the inhibition of p300 by ibuprofen-CoA, a known NSAID metabolite, reveals that linkage of ibuprofen to CoA increases its biochemical potency toward p300 and other KAT enzymes. In cellular studies, we find that carboxylate-containing NSAIDs inhibit histone acetylation. Finally, we exploit the stereoselective metabolism of ibuprofen to assess the role of its acyl-CoA metabolite in regulation of histone acetylation. This unique strategy reveals that formation of ibuprofen-CoA and histone acetylation are poorly correlated, suggesting metabolism may not be required for ibuprofen to inhibit histone acetylation. Overall, these studies provide new insights into the ability of NSAIDs to alter histone acetylation, and illustrate how selective metabolism may be leveraged as a tool to explore the influence of metabolic acyl-CoAs on cellular enzyme activity.
中文翻译:

通过NSAID化学型定义组蛋白乙酰化的代谢和非代谢调控。
非甾体抗炎药(NSAIDs)因其对炎症基因表达的作用而闻名。尽管已知NSAID会影响多种细胞信号传导机制,但最近的发现是NSAID水杨酸酯可部分抑制赖氨酸乙酰基转移酶(KAT)p300 / CBP破坏组蛋白乙酰化。水杨酸盐是一种相对较弱的KAT抑制剂,但其与CoA相连的代谢产物更有效。然而,NSAID代谢物在生物化学和细胞内抑制KAT酶的能力仍未得到开发。在这里,我们定义了新陈代谢和非代谢机制在NSAID化学型抑制KAT活性中的作用。首先,我们筛选了一小组NSAID,以抑制原型KAT p300的生化,从而发现许多含羧酸盐的NSAID,包括布洛芬在内,都可以起弱抑制剂的作用。评估布洛芬-CoA(一种已知的NSAID代谢产物)对p300的抑制作用表明,布洛芬与CoA的连接增加了其对p300和其他KAT酶的生化能力。在细胞研究中,我们发现含羧酸盐的NSAIDs抑制组蛋白乙酰化。最后,我们利用布洛芬的立体选择性代谢来评估其酰基辅酶A代谢产物在组蛋白乙酰化调节中的作用。这种独特的策略揭示了布洛芬-CoA的形成与组蛋白乙酰化的相关性很弱,这表明布洛芬抑制组蛋白乙酰化可能不需要代谢。总体而言,这些研究为NSAID改变组蛋白乙酰化的能力提供了新的见解,
更新日期:2017-12-14
中文翻译:

通过NSAID化学型定义组蛋白乙酰化的代谢和非代谢调控。
非甾体抗炎药(NSAIDs)因其对炎症基因表达的作用而闻名。尽管已知NSAID会影响多种细胞信号传导机制,但最近的发现是NSAID水杨酸酯可部分抑制赖氨酸乙酰基转移酶(KAT)p300 / CBP破坏组蛋白乙酰化。水杨酸盐是一种相对较弱的KAT抑制剂,但其与CoA相连的代谢产物更有效。然而,NSAID代谢物在生物化学和细胞内抑制KAT酶的能力仍未得到开发。在这里,我们定义了新陈代谢和非代谢机制在NSAID化学型抑制KAT活性中的作用。首先,我们筛选了一小组NSAID,以抑制原型KAT p300的生化,从而发现许多含羧酸盐的NSAID,包括布洛芬在内,都可以起弱抑制剂的作用。评估布洛芬-CoA(一种已知的NSAID代谢产物)对p300的抑制作用表明,布洛芬与CoA的连接增加了其对p300和其他KAT酶的生化能力。在细胞研究中,我们发现含羧酸盐的NSAIDs抑制组蛋白乙酰化。最后,我们利用布洛芬的立体选择性代谢来评估其酰基辅酶A代谢产物在组蛋白乙酰化调节中的作用。这种独特的策略揭示了布洛芬-CoA的形成与组蛋白乙酰化的相关性很弱,这表明布洛芬抑制组蛋白乙酰化可能不需要代谢。总体而言,这些研究为NSAID改变组蛋白乙酰化的能力提供了新的见解,











































 京公网安备 11010802027423号
京公网安备 11010802027423号