当前位置:
X-MOL 学术
›
J. Food Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Assessing the potential of whey protein fibril as emulsifier
Journal of Food Engineering ( IF 5.3 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.jfoodeng.2017.12.006 Raphaela Araujo Mantovani , Guilherme de Figueiredo Furtado , Flavia Maria Netto , Rosiane Lopes Cunha
Journal of Food Engineering ( IF 5.3 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.jfoodeng.2017.12.006 Raphaela Araujo Mantovani , Guilherme de Figueiredo Furtado , Flavia Maria Netto , Rosiane Lopes Cunha
|
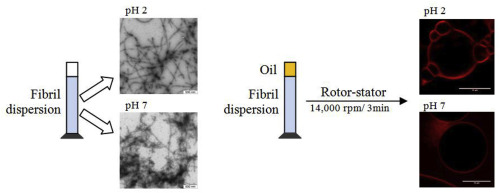
Abstract The effect of pH and mechanical processes on non-heated whey protein isolate (WPI) and WPI fibril was evaluated from structural, physical and emulsifying properties. Fibril aggregation was observed increasing pH from 2 to 7 whilst mechanical processes with high energy density resulted in fibril shortening and smaller aggregates. Despite affecting fibril morphology, mechanical processes did not affect significantly the protein secondary structure conformation and interfacial tension at aqueous protein dispersion-oil interface. However, increasing pH resulted in loss of ordered secondary structure, decreased interfacial tension and surface hydrophobicity of fibril. Fibril dispersions showed shear-thinning behavior but thixotropy was only observed at pH 7. Fibril-stabilized emulsions at pH 7 were more stable due to the higher viscosity and faster migration of fibrils to the interface when compared to fibrils at low pH, resulting in steric hindrance stabilization. Therefore, changes on WPI fibrils resulted in varied structural and emulsifying properties.
中文翻译:

评估乳清蛋白原纤维作为乳化剂的潜力
摘要 从结构、物理和乳化特性评估了 pH 值和机械过程对非加热乳清分离蛋白 (WPI) 和 WPI 原纤维的影响。观察到原纤维聚集使 pH 从 2 增加到 7,而具有高能量密度的机械过程导致原纤维缩短和更小的聚集体。尽管影响原纤维形态,机械过程并未显着影响蛋白质二级结构构象和水性蛋白质分散体-油界面的界面张力。然而,增加pH导致有序二级结构的丧失、原纤维的界面张力和表面疏水性降低。原纤维分散体表现出剪切稀化行为,但仅在 pH 值为 7 时观察到触变性。与低 pH 值的原纤维相比,pH 7 的原纤维稳定乳液更稳定,因为与低 pH 值的原纤维相比,原纤维具有更高的粘度和更快的迁移,导致空间位阻稳定。因此,WPI 原纤维的变化导致了不同的结构和乳化性能。
更新日期:2018-04-01
中文翻译:

评估乳清蛋白原纤维作为乳化剂的潜力
摘要 从结构、物理和乳化特性评估了 pH 值和机械过程对非加热乳清分离蛋白 (WPI) 和 WPI 原纤维的影响。观察到原纤维聚集使 pH 从 2 增加到 7,而具有高能量密度的机械过程导致原纤维缩短和更小的聚集体。尽管影响原纤维形态,机械过程并未显着影响蛋白质二级结构构象和水性蛋白质分散体-油界面的界面张力。然而,增加pH导致有序二级结构的丧失、原纤维的界面张力和表面疏水性降低。原纤维分散体表现出剪切稀化行为,但仅在 pH 值为 7 时观察到触变性。与低 pH 值的原纤维相比,pH 7 的原纤维稳定乳液更稳定,因为与低 pH 值的原纤维相比,原纤维具有更高的粘度和更快的迁移,导致空间位阻稳定。因此,WPI 原纤维的变化导致了不同的结构和乳化性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号