Organic Electronics ( IF 2.7 ) Pub Date : 2017-12-14 , DOI: 10.1016/j.orgel.2017.12.014 Zhen Xu , Lingqian Kong , Yunzhi Wang , Bozhen Wang , Jinsheng Zhao
|
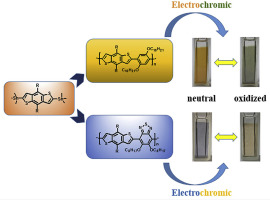
2,6-bis(trimethylstannane)-4,8-bis(2-octyldecyloxy)benzo[1,2-b:4,5-b′]dithiophene coupled with 2,5-didecyloxy-1,4-dibromobenzene and 4,7-dibromo-5,6-dioctyloxy-2,1,3-benzothiadiazole using Stille-coupling polymerization to afford the non D-A type polymer P1 and D-A type polymer P2, respectively. Two polymers displayed excellent solubility in normal organic solvents, so series of chemical instrumental analysis, such as NMR, GPC and elemental analysis, were carried out to confirm the inner structure and composition of the polymers. Different structure types exerted great influence on polymer's optical, electrochemical and electrochromic properties. Compared with P1 film, the introduction of the strong electron-withdrawing group benzothiadiazole improved conjugate effect along the polymer backbone of P2, which caused the redshift of ultraviolet–visible absorption spectrum and significant reduction in energy band gap. In addition, electrochromic performance test results showed that the P1 polymer film can switch between the orange-yellow and gray, while P2 film changed from blue in the neutral state to light yellow in the oxidation state. Moreover, the optical contrasts and response times of the polymer films also showed great differences due to the different molecular structure types. Higher transmittance changes and less sensitivity to the step interval on optical contrast indicated the fast switching speed and the superior cycle stability of P2 film.
中文翻译:

溶液可处理的基于BDT的电致变色材料中的带隙,色彩切换,光学对比度和氧化还原稳定性的调整
2,6-双(三甲基锡烷)-4,8-双(2-辛基癸氧基)苯并[1,2-b:4,5-b']二噻吩与2,5-二癸氧基-1,4-二溴苯和4使用Stille-偶联聚合法得到1,7-二溴-5,6-二辛氧基-2,1,3-苯并噻二唑,分别得到非DA型聚合物P1和DA型聚合物P2。两种聚合物在普通有机溶剂中均显示出优异的溶解性,因此进行了一系列化学仪器分析,如NMR,GPC和元素分析,以确认聚合物的内部结构和组成。不同的结构类型对聚合物的光学,电化学和电致变色性能影响很大。与P1薄膜相比,强吸电子基苯并噻二唑的引入改善了P2聚合物主链上的共轭效应,这导致了紫外可见吸收光谱的红移并显着减小了能带隙。此外,电致变色性能测试结果表明,P1聚合物膜可以在橙黄色和灰色之间切换,而P2膜从中性状态的蓝色变为氧化态的浅黄色。而且,由于不同的分子结构类型,聚合物膜的光学对比度和响应时间也显示出很大的差异。较高的透射率变化和对光学对比度的阶跃间隔的较小敏感性表明P2膜具有较快的转换速度和优异的循环稳定性。电致变色性能测试结果表明,P1聚合物膜可在橙黄色和灰色之间切换,而P2膜从中性状态的蓝色变为氧化态的浅黄色。此外,由于不同的分子结构类型,聚合物膜的光学对比度和响应时间也显示出很大的差异。更高的透射率变化和对光学对比度对步距间隔的较小敏感性表明P2膜具有更快的切换速度和出色的循环稳定性。电致变色性能测试结果表明,P1聚合物膜可在橙黄色和灰色之间切换,而P2膜从中性状态的蓝色变为氧化态的浅黄色。而且,由于不同的分子结构类型,聚合物膜的光学对比度和响应时间也显示出很大的差异。较高的透射率变化和对光学对比度的阶跃间隔的较小敏感性表明P2膜具有较快的转换速度和优异的循环稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号