当前位置:
X-MOL 学术
›
Fuel Process. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reductive carbonylation of methanol for ethanol production in Rh-Ru-dppp-methyl iodide catalytic system under mild conditions – The effect of lithium salts and catalyst composition
Fuel Processing Technology ( IF 7.2 ) Pub Date : 2018-03-01 , DOI: 10.1016/j.fuproc.2017.12.002 Yingzan Chen , Dianhua Liu
Fuel Processing Technology ( IF 7.2 ) Pub Date : 2018-03-01 , DOI: 10.1016/j.fuproc.2017.12.002 Yingzan Chen , Dianhua Liu
|
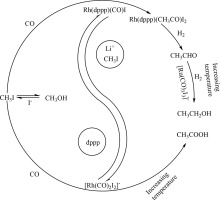
Abstract Reductive carbonylation of methanol is a potential process for ethanol production, but the reaction temperature is always higher than 190 °C and reaction pressure higher than 19 MPa. Due to the corrosions of the catalytic system, the reaction conditions, especially the pressure limited commercial applications of this process. The catalytic system consists of rhodium, ruthenium, dppp and methyl iodide, and was investigated for reductive carbonylation of methanol to ethanol under 6.0 MPa at 120 °C, and it exhibited an attractive catalytic activity. By comparing the products selectivity and the turnover frequency (TOF) of the catalytic systems, the role of each component in the catalytic system has been investigated. Rhodium catalyst was found to catalyze methanol reductive carbonylation for acetaldehyde formation, and ruthenium catalyst was responsible to catalyze hydrogenation of acetaldehyde to ethanol. The dppp can coordinate to rhodium and form a molecule rhodium active species, which enhanced the stability and solubility of the rhodium catalyst under the relatively low reaction pressure. The methyl iodide can promote the split of a carbon‑oxygen bond of methanol, thus accelerate the reaction process even at 120 °C. The synergy effects of these catalyst compositions give rise to the ethanol formation under the relatively mild condition. Additionally, reaction conditions, catalytic system proportional and presence of lithium salts were combined to tune the TOF and ethanol production. Properly increasing initial methyl iodide addition, dppp/Rh mole ratio, H 2 /CO mole ratio, reaction temperature and pressure can accelerate the TOF. Raising Ru/Rh ratio and reaction temperature were in favor of acetaldehyde hydrogenation to ethanol. High H 2 /CO and dppp/Rh ratio can suppress the acetic acid formation. This work could provide a deeper understanding for further optimization to enhanced ethanol production.
中文翻译:

Rh-Ru-dppp-甲基碘催化体系中温和条件下甲醇还原羰基化生产乙醇——锂盐和催化剂组成的影响
摘要 甲醇还原羰基化是乙醇生产的潜在工艺,但反应温度始终高于190 °C,反应压力高于19 MPa。由于催化体系的腐蚀,反应条件,特别是压力限制了该方法的商业应用。该催化体系由铑、钌、dppp 和碘甲烷组成,并在 6.0 MPa、120 °C 下研究了甲醇还原羰基化为乙醇的反应,并表现出有吸引力的催化活性。通过比较催化系统的产物选择性和周转频率(TOF),研究了每个组分在催化系统中的作用。发现铑催化剂催化甲醇还原羰基化反应生成乙醛,钌催化剂负责催化乙醛加氢制乙醇。dppp可以与铑配位形成分子铑活性物种,提高了铑催化剂在较低反应压力下的稳定性和溶解性。碘甲烷可以促进甲醇的碳氧键断裂,因此即使在 120 °C 下也能加速反应过程。这些催化剂组合物的协同作用导致在相对温和的条件下形成乙醇。此外,将反应条件、催化系统比例和锂盐的存在结合起来调整 TOF 和乙醇的生产。适当增加初始甲基碘添加量、dppp/Rh 摩尔比、H 2 /CO 摩尔比、反应温度和压力可以加速TOF。提高Ru/Rh比和反应温度有利于乙醛加氢制乙醇。高 H 2 /CO 和 dppp/Rh 比值可以抑制乙酸的形成。这项工作可以为进一步优化以提高乙醇生产提供更深入的理解。
更新日期:2018-03-01
中文翻译:

Rh-Ru-dppp-甲基碘催化体系中温和条件下甲醇还原羰基化生产乙醇——锂盐和催化剂组成的影响
摘要 甲醇还原羰基化是乙醇生产的潜在工艺,但反应温度始终高于190 °C,反应压力高于19 MPa。由于催化体系的腐蚀,反应条件,特别是压力限制了该方法的商业应用。该催化体系由铑、钌、dppp 和碘甲烷组成,并在 6.0 MPa、120 °C 下研究了甲醇还原羰基化为乙醇的反应,并表现出有吸引力的催化活性。通过比较催化系统的产物选择性和周转频率(TOF),研究了每个组分在催化系统中的作用。发现铑催化剂催化甲醇还原羰基化反应生成乙醛,钌催化剂负责催化乙醛加氢制乙醇。dppp可以与铑配位形成分子铑活性物种,提高了铑催化剂在较低反应压力下的稳定性和溶解性。碘甲烷可以促进甲醇的碳氧键断裂,因此即使在 120 °C 下也能加速反应过程。这些催化剂组合物的协同作用导致在相对温和的条件下形成乙醇。此外,将反应条件、催化系统比例和锂盐的存在结合起来调整 TOF 和乙醇的生产。适当增加初始甲基碘添加量、dppp/Rh 摩尔比、H 2 /CO 摩尔比、反应温度和压力可以加速TOF。提高Ru/Rh比和反应温度有利于乙醛加氢制乙醇。高 H 2 /CO 和 dppp/Rh 比值可以抑制乙酸的形成。这项工作可以为进一步优化以提高乙醇生产提供更深入的理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号