当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evidence for Dynamic Chemical Kinetics at Individual Molecular Ruthenium Catalysts
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-09 , DOI: 10.1002/anie.201711314 Quinn T. Easter 1 , Suzanne A. Blum 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-09 , DOI: 10.1002/anie.201711314 Quinn T. Easter 1 , Suzanne A. Blum 1
Affiliation
|
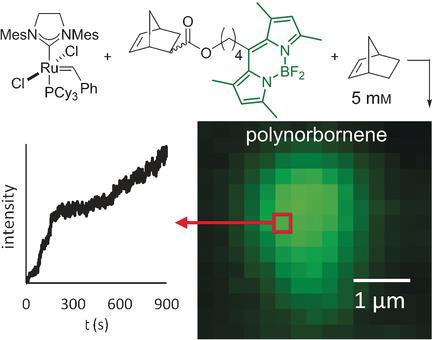
Catalytic cycles are typically depicted as possessing time‐invariant steps with fixed rates. Yet the true behavior of individual catalysts with respect to time is unknown, hidden by the ensemble averaging inherent to bulk measurements. Evidence is presented for variable chemical kinetics at individual catalysts, with a focus on ring‐opening metathesis polymerization catalyzed by the second‐generation Grubbs’ ruthenium catalyst. Fluorescence microscopy is used to probe the chemical kinetics of the reaction because the technique possesses sufficient sensitivity for the detection of single chemical reactions. Insertion reactions in submicron regions likely occur at groups of many (not single) catalysts, yet not so many that their unique kinetic behavior is ensemble averaged.
中文翻译:

个别分子钌催化剂上动态化学动力学的证据
催化循环通常被描述为具有固定速率的时不变步骤。然而,各个催化剂相对于时间的真实行为是未知的,被整体测量固有的整体平均所掩盖。给出了各种催化剂动力学化学动力学的证据,重点是由第二代Grubbs钌催化剂催化的开环易位聚合。荧光显微镜用于探测反应的化学动力学,因为该技术对于检测单个化学反应具有足够的灵敏度。亚微米区域中的插入反应可能发生在许多(不是单一)催化剂的组中,但数量不多,以至于其独特的动力学行为是整体平均的。
更新日期:2018-01-09
中文翻译:

个别分子钌催化剂上动态化学动力学的证据
催化循环通常被描述为具有固定速率的时不变步骤。然而,各个催化剂相对于时间的真实行为是未知的,被整体测量固有的整体平均所掩盖。给出了各种催化剂动力学化学动力学的证据,重点是由第二代Grubbs钌催化剂催化的开环易位聚合。荧光显微镜用于探测反应的化学动力学,因为该技术对于检测单个化学反应具有足够的灵敏度。亚微米区域中的插入反应可能发生在许多(不是单一)催化剂的组中,但数量不多,以至于其独特的动力学行为是整体平均的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号