Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2017-12-06 , DOI: 10.1016/j.jcou.2017.12.003 Jing Peng , Hai-Jian Yang , Sheng Wang , Binru Ban , Zidong Wei , Bo Lei , Cun-Yue Guo
|
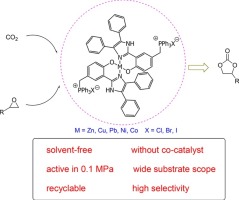
A series of metal complexes containing a metal ion (Zn, Cu, Pb, Ni, Co) and two quaternary phosphonium salt units anchored on the ligands were designed and synthesized as efficient single-component bifunctional catalyst for the solvent-free coupling reaction of CO2 and epoxides without any co-catalysts. The effects of various reaction variables on the catalytic activity were investigated systematically and the optimized reaction conditions were screened as (130 °C, 1 MPa and 5 h). The order of the catalytic activity for these single-component bifunctional metal complexes was found to be Zn-PPBCl (96%) > Co-PPBCl (90%) ≈ Ni-PPBCl (90%) > Pb-PPBCl (86%) > Cu-PPBCl (15%). Notably, a high turnover frequency (TOF) value (1230 h−1) for bifunctional catalyst Zn-PPBCl was achieved via adjusting reaction variables. Moreover, this series of catalysts can also catalyze the coupling reaction at atmospheric pressure, and most of them showed high conversion of epoxide (propylene carbonate (PC) yield > 90%) within 7 h. The catalysts are also applicable to a variety of epoxides and good catalytic performances were achieved for producing the corresponding cyclic carbonates in most cases. Furthermore, the catalyst can be easily recycled and reused for at least five times without dramatic activity loss after simple filtration and drying. Finally, kinetic studies were carried out preliminarily for four metal (Zn, Co, Ni, Pb) catalysts and the formation activation energies (Ea) of cyclic carbonate were obtained. The apparent activation energy Ea catalyzed by Zn-PPBCl is only 37.6 kJ/mol, while the Ea (Pb-PPBCl) is 70.0 kJ/mol, Ea (Ni-PPBCl) is 65.6 kJ/mol, and Ea (Co-PPBCl) is 43.1 kJ/mol. The sequence of Ea agrees well with the catalytic activity. Based on the kinetic studies and experimental results, an inferred mechanism was deduced.
中文翻译:

新型可回收的双功能金属配合物催化的高效无溶剂固定CO 2
设计并合成了一系列包含金属离子(Zn,Cu,Pb,Ni,Co)和两个固定在配体上的季phospho盐单元的金属配合物,作为有效的单组分双功能催化剂,用于CO的无溶剂偶联反应2和环氧化物,无任何助催化剂。系统地研究了各种反应变量对催化活性的影响,筛选出最佳反应条件为(130℃,1MPa和5h)。发现这些单组分双功能金属络合物的催化活性顺序为Zn-PPBCl(96%)> Co-PPBCl(90%)≈Ni-PPBCl(90%)> Pb-PPBCl(86%)>铜-PPBCl(15%)。值得注意的是,实现了双功能催化剂Zn-PPBCl的高周转频率(TOF)值(1230 h -1)通过调整反应变量。此外,该系列催化剂还可以在大气压下催化偶联反应,并且大多数催化剂在7小时内显示出高的环氧化物转化率(碳酸亚丙酯(PC)产率> 90%)。所述催化剂也适用于多种环氧化物,并且在大多数情况下获得了用于生产相应的环状碳酸酯的良好催化性能。此外,在简单过滤和干燥后,该催化剂可以容易地再循环和再使用至少五次,而不会显着降低活性。最后,对四种金属(Zn,Co,Ni,Pb)催化剂进行了初步的动力学研究,得到了环状碳酸酯的形成活化能(E a)。表观活化能E aZn-PPBCl催化仅为37.6 kJ / mol,而E a(Pb-PPBCl)为70.0 kJ / mol,E a(Ni-PPBCl)为65.6 kJ / mol,E a(Co-PPBCl)为43.1 kJ /摩尔 E a的序列与催化活性非常吻合。在动力学研究和实验结果的基础上,推导了机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号