当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Molecular Structure of gauche‐1,3‐Butadiene: Experimental Establishment of Non‐planarity
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-18 , DOI: 10.1002/anie.201709966 Joshua H. Baraban 1, 2 , Marie-Aline Martin-Drumel 3, 4 , P. Bryan Changala 5 , Sandra Eibenberger 6 , Matthew Nava 7, 8 , David Patterson 6, 9 , John F. Stanton 10 , G. Barney Ellison 1 , Michael C. McCarthy 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-18 , DOI: 10.1002/anie.201709966 Joshua H. Baraban 1, 2 , Marie-Aline Martin-Drumel 3, 4 , P. Bryan Changala 5 , Sandra Eibenberger 6 , Matthew Nava 7, 8 , David Patterson 6, 9 , John F. Stanton 10 , G. Barney Ellison 1 , Michael C. McCarthy 3
Affiliation
|
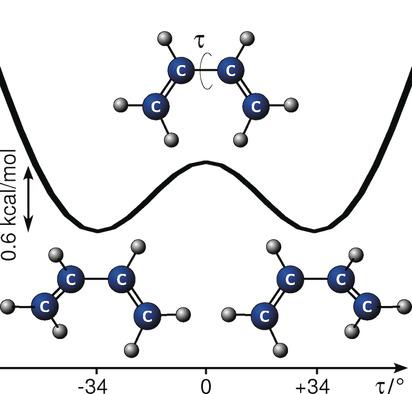
The planarity of the second stable conformer of 1,3‐butadiene, the archetypal diene for the Diels–Alder reaction in which a planar conjugated diene and a dienophile combine to form a ring, is not established. The most recent high level calculations predicted the species to adopt a twisted, gauche structure owing to steric interactions between the inner terminal hydrogens rather than a planar, cis structure favored by the conjugation of the double bonds. The structure cis‐1,3‐butadiene is unambiguously confirmed experimentally to indeed be gauche with a substantial dihedral angle of 34°, in excellent agreement with theory. Observation of two tunneling components indicates that the molecule undergoes facile interconversion between two equivalent enantiomeric forms. Comparison of experimentally determined structures for gauche‐ and trans‐butadiene provides an opportunity to examine the effects of conjugation and steric interactions.
中文翻译:

gauche-1,3-丁二烯的分子结构:非平面性的实验建立
没有建立第二稳定构型的1,3-丁二烯的平面性,即Diels-Alder反应的原型二烯,其中平面共轭二烯和亲二烯体结合形成环。最新的高水平计算预测,由于内部末端氢原子之间的空间相互作用,该物种将采用扭曲的gauche结构,而不是受到双键共轭作用的平面顺式结构。实验明确地确定了顺式1,3-丁二烯的结构确实是薄纱,其二面角为34°,与理论非常吻合。对两个隧穿组分的观察表明,该分子在两种等同的对映体形式之间进行了容易的相互转化。
更新日期:2018-01-18
中文翻译:

gauche-1,3-丁二烯的分子结构:非平面性的实验建立
没有建立第二稳定构型的1,3-丁二烯的平面性,即Diels-Alder反应的原型二烯,其中平面共轭二烯和亲二烯体结合形成环。最新的高水平计算预测,由于内部末端氢原子之间的空间相互作用,该物种将采用扭曲的gauche结构,而不是受到双键共轭作用的平面顺式结构。实验明确地确定了顺式1,3-丁二烯的结构确实是薄纱,其二面角为34°,与理论非常吻合。对两个隧穿组分的观察表明,该分子在两种等同的对映体形式之间进行了容易的相互转化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号