Acta Biomaterialia ( IF 9.4 ) Pub Date : 2017-12-12 , DOI: 10.1016/j.actbio.2017.12.003 Xiaoming Luo , Maohua Chen , Zhoujiang Chen , Songzhi Xie , Nan He , Tao Wang , Xiaohong Li
|
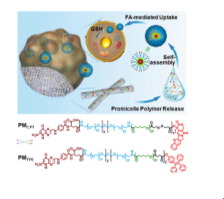
Camptothecin (CPT)-containing promicelle polymers (PMCPT) based on 4-armed poly(ethylene glycol) (PEG) were developed previously to self-assemble into folate-targeted and glutathione (GSH)-sensitive micelles (MCPT). To address severe systemic toxicity and lack of tumor specificity implicated in the intravenous administration of MCPT, a micelle-generating depot has been developed by blend electrospinning of PEG-poly(lactide) (PELA) copolymers, PMCPT and polyethylene oxide (PEO). Upon implantation of the depot onto the tumor, PMCPT are sustainably released to spontaneously form MCPT on the tumor site. The release of PMCPT is adjusted by varying PEO/PELA ratios and reach in the range of 23−92% after 30 days of incubation in PBS. By making use of the aggregation-induced emission (AIE) features of tetraphenylethylene (TPE) derivatives, the release process of TPE-containing promicelle polymers (PMTPE) from the depot and the formation of micelles (MTPE) have been monitored from the self-assembly-induced fluorescence light-up both in vitro and in vivo. Compared with intravenous injection of MCPT, the micelle-generating depot has significantly enhanced micelle accumulation at tumor sites for an extended period of time and resulted in stronger tumor inhibitory efficacy, reduced systemic toxicity and more effective inhibition of tumor metastasis, demonstrating great potential for targeted cancer therapy with sustained efficacy.
Statement of Significance
The promicelle polymer-co-electrospun fibers are developed to form a micelle-generating depot after implantation onto the tumor. The promicelle polymers are continuously released and simultaneously self-assemble into folate-targeted and glutathione-sensitive micelles, ensuring sustained micelle delivery for more than 30 days. The process of micelle formation at tumor site is visualized in vivo for the first time based on the mechanism of aggregation-induced emission. This in situ micelle formation also prevents premature drug release and rapid clearance from the bloodstream. In addition, these fibers deliver anti-cancer agents directly within tumor cells via dual selectivity (i.e. spatially selective accumulation in tumor tissues via implantation and selective internalization into tumor cells via folate receptor-mediated endocytosis) and on-demand drug release in response to cytosol GSH. They exhibit superior tumor inhibitory efficacy with minimal systemic toxicity, and prevent from malignant metastasis of cancer cells.
中文翻译:

一种能够原位产生胶束以实现受控和靶向的肿瘤化学疗法的可植入储库
基于四臂聚乙二醇(PEG )的含有喜树碱(CPT)的束状聚合物(PM CPT)先前已经开发出来,可以自组装成叶酸靶向和谷胱甘肽(GSH)敏感的胶束(MCPT)。为了解决静脉注射M CPT所引起的严重的全身毒性和缺乏肿瘤特异性,已经通过将PEG-聚(丙交酯)(PELA)共聚物,PM CPT和聚环氧乙烷(PEO)混合电纺丝开发了一种产生胶束的贮库。。在将贮库植入肿瘤上时,PM CPT被持续释放以在肿瘤部位自发形成M CPT。PM CPT的发布通过改变PEO / PELA比例可调节PBS,在PBS中孵育30天后,可达到23-92%的范围。通过利用四苯乙烯(TPE)衍生物的聚集诱导发射(AIE)功能,从聚苯乙烯中监测了含TPE的胶束聚合物(PM TPE)从储库的释放过程和胶束(M TPE)的形成过程。自组装诱导荧光点亮二者在体外和体内。与静脉注射M CPT相比,产生胶束的贮藏库在延长的时间段内显着增强了胶束在肿瘤部位的蓄积,并导致更强的肿瘤抑制功效,降低的全身毒性和对肿瘤转移的更有效抑制,显示了具有持续疗效的靶向癌症治疗的巨大潜力。
重要声明
胶束聚合物-共电纺丝纤维在植入肿瘤后被显影以形成胶束生成贮库。胶束聚合物被连续释放并同时自组装成叶酸靶向和谷胱甘肽敏感的胶束,从而确保胶束持续递送超过30天。基于聚集诱导的发射机制,首次在体内可视化了肿瘤部位的胶束形成过程。这种原位胶束的形成还防止了药物的过早释放和从血流中的快速清除。另外,这些纤维通过双重选择性(即在肿瘤组织中的空间选择性积累)直接在肿瘤细胞内递送抗癌药。通过注入和选择性内化至肿瘤细胞通过响应叶酸受体介导的内吞作用)和按需药物释放到胞浆GSH。它们表现出优异的肿瘤抑制功效,具有最小的全身毒性,并可以防止癌细胞的恶性转移。











































 京公网安备 11010802027423号
京公网安备 11010802027423号