Acta Biomaterialia ( IF 9.4 ) Pub Date : 2017-12-08 , DOI: 10.1016/j.actbio.2017.11.052 Matthew I Converse 1 , Raymond G Walther 1 , Justin T Ingram 2 , Yang Li 2 , S Michael Yu 3 , Kenneth L Monson 4
|
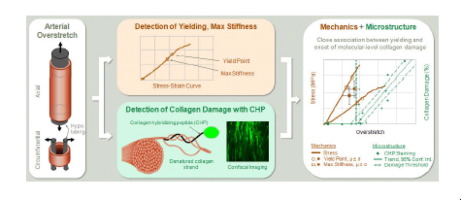
It is well established that overstretch of arteries alters their mechanics and compromises their function. However, the underlying structural mechanisms behind these changes are poorly understood. Utilizing a recently developed collagen hybridizing peptide (CHP), we demonstrate that a single mechanical overstretch of an artery produces molecular-level unfolding of collagen. In addition, imaging and quantification of CHP binding revealed that overstretch produces damage (unfolding) among fibers aligned with the direction of loading, that damage increases with overstretch severity, and that the onset of this damage is closely associated with tissue yielding. These findings held true for both axial and circumferential loading directions. Our results are the first to identify stretch-induced molecular damage to collagen in blood vessels. Furthermore, our approach is advantageous over existing methods of collagen damage detection as it is non-destructive, readily visualized, and objectively quantified. This work opens the door to revealing additional structure-function relationships in arteries. We anticipate that this approach can be used to better understand arterial damage in clinically relevant settings such as angioplasty and vascular trauma. Furthermore, CHP can be a tool for the development of microstructurally-based constitutive models and experimentally validated computational models of arterial damage and damage propagation across physical scales.
Statement of Significance
Arteries play a critical role by carrying oxygen and essential nutrients throughout the body. However, trauma to the head and neck, as well as surgical interventions, can overstretch arteries and alter their mechanics. In order to better understand the cause of these changes, we employ a novel collagen hybridizing peptide (CHP) to study collagen damage in overstretched arteries. Our approach is unique in that we go beyond the fiber- and fibril-level and characterize molecular-level disruption. In addition, we image and quantify fluorescently-labeled CHP to reveal a new structure-property relationship in arterial damage. We anticipate that our approach can be used to better understand arterial damage in clinically relevant settings such as angioplasty and vascular trauma.
中文翻译:

过度拉伸的脑动脉中分子水平胶原蛋白损伤的检测和表征。
众所周知,动脉过度伸展会改变其力学并损害其功能。然而,人们对这些变化背后的潜在结构机制知之甚少。利用最近开发的胶原蛋白杂交肽(CHP),我们证明了动脉的单次机械过度拉伸会产生分子水平的胶原蛋白展开。此外,CHP 结合的成像和定量显示,过度拉伸会在与负载方向对齐的纤维中产生损伤(展开),损伤随着过度拉伸的严重程度而增加,并且这种损伤的发生与组织屈服密切相关。这些发现对于轴向和周向载荷方向都适用。我们的结果首次确定了拉伸引起的血管胶原蛋白分子损伤。此外,我们的方法优于现有的胶原蛋白损伤检测方法,因为它是非破坏性的、易于可视化和客观量化的。这项工作为揭示动脉中其他结构与功能的关系打开了大门。我们预计这种方法可用于更好地了解临床相关环境中的动脉损伤,例如血管成形术和血管创伤。此外,CHP 可以成为开发基于微观结构的本构模型和经过实验验证的动脉损伤和跨物理尺度损伤传播的计算模型的工具。
重要性声明
动脉通过将氧气和必需营养物质输送到全身而发挥着至关重要的作用。然而,头部和颈部的创伤以及手术干预可能会过度拉伸动脉并改变其力学结构。为了更好地了解这些变化的原因,我们采用一种新型胶原蛋白杂交肽(CHP)来研究过度拉伸动脉中的胶原蛋白损伤。我们的方法的独特之处在于,我们超越了纤维和原纤维水平,并表征了分子水平的破坏。此外,我们对荧光标记的 CHP 进行成像和量化,以揭示动脉损伤中新的结构-性质关系。我们预计我们的方法可用于更好地了解临床相关环境中的动脉损伤,例如血管成形术和血管创伤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号