Molecular and Cellular Neuroscience ( IF 2.6 ) Pub Date : 2017-12-12 , DOI: 10.1016/j.mcn.2017.12.005 Ningjing Huang , Christine Erie , Michael L. Lu , Jianning Wei
|
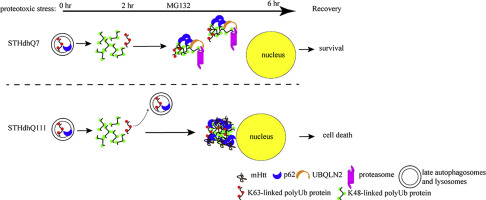
Proteotoxic stress plays an important role in the pathogenesis of Huntington's disease (HD). Autophagy is proposed as a compensatory mechanism to remove protein aggregates under proteotoxic stress by up-regulating p62 expression. In the present study, we investigated the molecular action of p62 to proteotoxic stress in HD cells. Using two different HD cellular models, STHdhQ7 and STHdhQ111 cells derived from wild type and HD knock-in mice and human fibroblasts from healthy and HD patients, we found that HD cells are more vulnerable to cell death under proteotoxic stress and during stress recovery. We further showed that P62 was up-regulated in both STHdhQ7 and STHdhQ111 cells in response to the stress with distinct subcellular localization patterns. While dispersed p62 puncti were found in STHdhQ7 cells, p62 bodies were initially present in the lysosomes and accumulated to the juxtanuclear regions of STHdhQ111 cells as MG132 incubation continued. Unlike in STHdhQ7 cells, p62 puncti were not associated with K48-linked polyubiquitinated protein aggregates or proteasomal components in STHdhQ111. Interestingly, addition of cysteine during MG132 incubation rescued cell death in STHdhQ111 cells caused by stress recovery and altered the subcellular distribution of p62. Our data suggest that aberrant positioning of p62 affects the proteasomal clearance of protein aggregates and may contribute to the increased vulnerability to proteotoxic stress-induced cell death in HD cells.
中文翻译:

SQSTM1 / p62的异常亚细胞定位有助于增加对亨廷顿氏病蛋白毒性应激恢复的脆弱性
蛋白毒性应激在亨廷顿舞蹈病(HD)的发病机理中起着重要作用。自噬被认为是通过上调p62表达在蛋白毒性应激下去除蛋白质聚集体的一种补偿机制。在本研究中,我们调查了p62对HD细胞蛋白毒性应激的分子作用。使用两种不同的高清细胞模型,即来自野生型和高清敲入小鼠的STHdhQ7和STHdhQ111111细胞,以及来自健康和HD患者的人类成纤维细胞,我们发现HD细胞在蛋白毒性应激和应激恢复期间更容易死亡。我们进一步表明,响应于具有不同亚细胞定位模式的应激,STHdhQ7和STHdhQ111细胞中的P62均上调。虽然在STHdhQ7细胞中发现了分散的p62点,p62体最初存在于溶酶体中,并随着MG132继续孵育而积累到STHdhQ111细胞的近核区域。与STHdhQ7细胞不同,p62点与STHdhQ111中的K48连接的多泛素化蛋白聚集体或蛋白酶体组分无关。有趣的是,在MG132孵育过程中添加半胱氨酸可挽救STHdhQ111细胞因应力恢复而导致的细胞死亡,并改变p62的亚细胞分布。我们的数据表明,p62的异常定位会影响蛋白聚集体的蛋白酶体清除,并且可能导致对HD细胞中蛋白毒性应激诱导的细胞死亡的脆弱性增加。p62点与STHdhQ111中的K48连接的多泛素化蛋白聚集体或蛋白酶体成分无关。有趣的是,在MG132孵育过程中添加半胱氨酸可挽救STHdhQ111细胞因应力恢复而导致的细胞死亡,并改变p62的亚细胞分布。我们的数据表明,p62的异常定位会影响蛋白聚集体的蛋白酶体清除,并且可能导致对HD细胞中蛋白毒性应激诱导的细胞死亡的脆弱性增加。p62点与STHdhQ111中的K48连接的多泛素化蛋白聚集体或蛋白酶体成分无关。有趣的是,在MG132孵育过程中添加半胱氨酸可挽救STHdhQ111细胞因应力恢复而导致的细胞死亡,并改变p62的亚细胞分布。我们的数据表明,p62的异常定位会影响蛋白聚集体的蛋白酶体清除,并且可能导致对HD细胞中蛋白毒性应激诱导的细胞死亡的脆弱性增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号