Microporous and Mesoporous Materials ( IF 4.8 ) Pub Date : 2017-12-12 , DOI: 10.1016/j.micromeso.2017.12.011 Renju Zacharia , Luis Fernando Gomez , Richard Chahine , Daniel Cossement , Pierre Benard
|
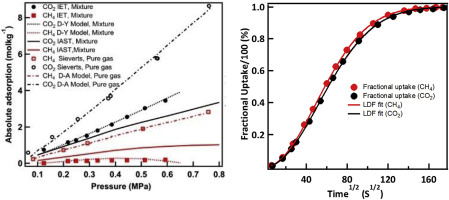
We combine the isotope exchange technique (IET) with the conventional Sieverts method, microcalorimetry, breakthrough experiments and multi-scale modelling to comprehensively and quantitatively investigate the thermodynamics and kinetics of equimolar CH4/CO2 mixture separation in metal organic frameworks. The prototypical MOF-5 is selected for this work as it allows benchmarking our binary mixture results with the pure gas data widely reported in the literature. For the first time, an experimental binary gas adsorption isotherm of CH4/CO2 on MOF-5 is reported and compared with the respective pure gas isotherms. The equilibrium thermodynamic selectivity from the IET experiments for the equimolar CH4/CO2 separation is found to be 8.3 while a much lower value of 2.83 is obtained from the ideal adsorption solution theory (IAST). The large standard deviation of the model selectivities and the significant deviation of averaged model selectivity from the experimental one clearly reinforces the necessity to determine the selectivity reliably using experiments. The kinetic selectivity for the binary mixture separation determined by combining the results of IET with the linear driving force (LDF) model is 0.73. The co-adsorption heats and excess uptake of both gases in mixtures are lower than those of pure gases; we observe that the intrinsically weaker sorption of CH4 on MOF-5 is further weakened by the presence of strongly interacting CO2. Thermodynamic and kinetic selectivities and the co-adsorption heats quantitatively suggests that CH4/CO2 separation is driven by the equilibrium thermodynamic factors with no significant contribution from kinetic factors.
中文翻译:

金属-有机骨架通过同位素交换和吸附突破分离CH 4 / CO 2二元混合物的热力学和动力学
我们将同位素交换技术(IET)与常规的Sieverts方法,微量量热法,突破性实验和多尺度建模相结合,以全面定量地研究金属有机骨架中等摩尔CH 4 / CO 2混合物分离的热力学和动力学。选择典型的MOF-5进行这项工作是因为它可以用文献中广泛报道的纯气体数据对我们的二元混合物结果进行基准测试。首次报道了MOF-5上CH 4 / CO 2的实验性二元气体吸附等温线,并与相应的纯气体等温线进行了比较。来自IET实验的等摩尔CH 4 / CO平衡热力学选择性发现2分离为8.3,而理想吸附溶液理论(IAST)则得到的分离度低得多,为2.83。模型选择性的大标准偏差和平均模型选择性与实验值的显着偏差明显加强了使用实验可靠地确定选择性的必要性。通过将IET结果与线性驱动力(LDF)模型相结合确定的二元混合物分离的动力学选择性为0.73。混合物中两种气体的共吸附热和过量吸收均低于纯气体。我们观察到,由于强烈相互作用的CO 2的存在,CH 4在MOF-5上的固有较弱的吸附作用进一步减弱。。热力学和动力学选择性以及共吸附热定量地表明,CH 4 / CO 2的分离是由平衡热力学因素驱动的,而动力学因素没有显着贡献。











































 京公网安备 11010802027423号
京公网安备 11010802027423号