Journal of the American Society for Mass Spectrometry ( IF 3.2 ) Pub Date : 2017-09-21 , DOI: 10.1007/s13361-017-1806-9 Hanxue Xia 1 , Athula B. Attygalle 1
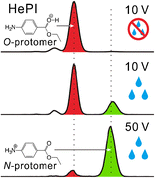
The role of water vapor in transforming the thermodynamically preferred species of protonated benzocaine to the less favored protomer was investigated using helium-plasma ionization (HePI) in conjunction with ion-mobility mass spectrometry (IM-MS). The IM arrival-time distribution (ATD) recorded from a neat benzocaine sample desorbed to the gas phase by a stream of dry nitrogen and ionized by HePI showed essentially one peak for the O-protonated species. However, when water vapor was introduced to the enclosed ion source, within a span of about 150 ms the ATD profile changed completely to one dominated by the N-protonated species. Under spray-based ionization conditions, the nature and composition of the solvents have been postulated to play a decisive role in defining the manifested protomer ratios. In reality, the solvent vapors present in the ion source (particularly the ambient humidity) indirectly dictate the gas-phase ratio of the protomers. Evidently, the gas-phase protomer ratio established at the confinement of the ions is readjusted by the ion-activation that takes place during the transmission of ions to the vacuum. Although it has been repeatedly stated that ions can retain a “memory” of their solution structures because they can be kinetically trapped, and thereby represent their solution-based stabilities, we show that the initial airborne ions can undergo significant transformations in the transit through the intermediate vacuum zones between the ion source and the mass detector. In this context, we demonstrate that the kinetically trapped N-protomer of benzocaine can be untrapped by reducing the humidity of the enclosed ion source.
ᅟ
中文翻译:

捕获动力学困住的离子:离子淌度质谱揭示的水蒸气和离子源活化条件对苯佐卡因的气相前驱体比率的作用。
使用氦等离子体电离(HePI)结合离子迁移质谱(IM-MS),研究了水蒸气在将热力学上优选的质子化苯并卡因物种转化为较不受欢迎的前列腺素中的作用。从通过干燥氮气流解吸到气相并被HePI电离的纯苯并卡因样品中记录的IM到达时间分布(ATD)基本上显示了一个O质子化的峰。但是,当将水蒸气引入封闭的离子源时,在约150 ms的跨度内,ATD曲线完全变为N质子化的物种。在基于喷雾的电离条件下,已假定溶剂的性质和组成在定义所显示的protomer比率中起决定性作用。实际上,离子源中存在的溶剂蒸汽(特别是环境湿度)间接决定了原质子的气相比。显然,通过将离子传输到真空过程中发生的离子活化作用,可以重新调节在离子限制范围内建立的气相质子比。尽管已经反复说明离子可以保留其溶液结构的“记忆”,因为它们可以被动力学捕获,从而代表其基于溶液的稳定性,我们表明,初始的空气中离子在穿过离子源和质量检测器之间的中间真空区的过程中会发生重大转变。在这种情况下,我们证明了可以通过降低封闭离子源的湿度来释放苯并卡因的动力学捕集的N-启动子。

ᅟ



























 京公网安备 11010802027423号
京公网安备 11010802027423号