当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational studies of Ant–Pro motif-incorporated cyclic peptides: gramicidin S and avellanin†
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2017-12-13 00:00:00 , DOI: 10.1039/c7nj03701e Amol S. Kotmale 1, 2, 3, 4, 5 , Ekta Sangtani 2, 3, 4, 6 , Rajesh G. Gonnade 2, 3, 4, 6 , Dhiman Sarkar 4, 7, 8, 9, 10 , Sachin Burade 4, 11, 12, 13 , Pattuparambil R. Rajamohanan 2, 3, 4, 5 , Gangadhar J. Sanjayan 1, 2, 3, 4
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2017-12-13 00:00:00 , DOI: 10.1039/c7nj03701e Amol S. Kotmale 1, 2, 3, 4, 5 , Ekta Sangtani 2, 3, 4, 6 , Rajesh G. Gonnade 2, 3, 4, 6 , Dhiman Sarkar 4, 7, 8, 9, 10 , Sachin Burade 4, 11, 12, 13 , Pattuparambil R. Rajamohanan 2, 3, 4, 5 , Gangadhar J. Sanjayan 1, 2, 3, 4
Affiliation
|
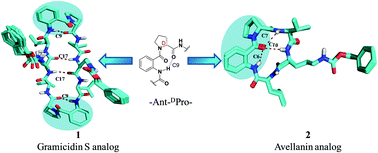
This paper reports conformational changes observed in cyclic bioactive peptides such as gramicidin S and avellanin upon incorporation of a pseudo-β (C9) Ant–DPro turn motif in their structural frameworks. Solution-state studies suggested that a synthetic gramicidin S analog exhibits a β-sheet conformation with C9 and C17 intramolecular hydrogen bonding patterns, while its truncated analog disturbs the β-sheet conformation. Structural details were obtained using a combination of CD studies, X-ray crystal structure studies and nOe-based MD simulation studies.
中文翻译:

Ant-Pro基序并入的环肽的构象研究:短杆菌肽S和avellanin †
本文报道了在其结构框架中掺入伪-β(C9)Ant– D Pro转位基序后,在诸如短杆菌肽S和avellanin等环状生物活性肽中观察到的构象变化。溶液状态研究表明,合成的短杆菌肽S类似物表现出具有C9和C17分子内氢键模式的β-折叠构象,而其截短的类似物干扰了β-折叠构象。通过结合CD研究,X射线晶体结构研究和基于nOe的MD模拟研究获得结构细节。
更新日期:2017-12-13
中文翻译:

Ant-Pro基序并入的环肽的构象研究:短杆菌肽S和avellanin †
本文报道了在其结构框架中掺入伪-β(C9)Ant– D Pro转位基序后,在诸如短杆菌肽S和avellanin等环状生物活性肽中观察到的构象变化。溶液状态研究表明,合成的短杆菌肽S类似物表现出具有C9和C17分子内氢键模式的β-折叠构象,而其截短的类似物干扰了β-折叠构象。通过结合CD研究,X射线晶体结构研究和基于nOe的MD模拟研究获得结构细节。











































 京公网安备 11010802027423号
京公网安备 11010802027423号