当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Simulation of Vapor-Liquid Equilibria Using the Wolf Method for Electrostatic Interactions.
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2017-12-13 , DOI: 10.1021/acs.jced.7b00839 Remco Hens 1 , Thijs J H Vlugt 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2017-12-13 , DOI: 10.1021/acs.jced.7b00839 Remco Hens 1 , Thijs J H Vlugt 1
Affiliation
|
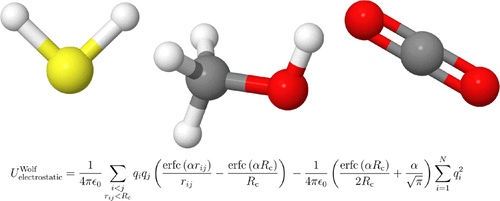
The applicability of the Wolf method for calculating electrostatic interactions is verified for simulating vapor-liquid equilibria of hydrogen sulfide, methanol, and carbon dioxide. Densities, chemical potentials, and critical properties are obtained with Monte Carlo simulations using the Continuous Fractional Component version of the Gibbs Ensemble. Saturated vapor pressures are obtained from NPT simulations. Excellent agreement is found between simulation results and data from literature (simulations using the Ewald summation). It is also shown how to choose the optimal parameters for the Wolf method. Even though the Wolf method requires a large simulation box in the gas phase, due to the lack of screening of electrostatics, one can consider the Wolf method as a suitable alternative to the Ewald summation in VLE calculations.
中文翻译:

使用沃尔夫方法进行静电相互作用的汽液平衡分子模拟。
为了模拟硫化氢,甲醇和二氧化碳的气-液平衡,验证了沃尔夫方法用于计算静电相互作用的适用性。密度,化学势和临界性质是使用Gibbs合奏的连续分数分量版本通过Monte Carlo模拟获得的。饱和蒸气压是从NPT模拟获得的。在仿真结果与文献数据(使用Ewald求和的仿真)之间发现了极好的一致性。还显示了如何为Wolf方法选择最佳参数。尽管沃尔夫方法需要在气相中使用大型仿真箱,但由于缺少静电屏蔽,因此人们可以将沃尔夫方法视为VLE计算中Ewald求和的一种合适替代方法。
更新日期:2017-12-13
中文翻译:

使用沃尔夫方法进行静电相互作用的汽液平衡分子模拟。
为了模拟硫化氢,甲醇和二氧化碳的气-液平衡,验证了沃尔夫方法用于计算静电相互作用的适用性。密度,化学势和临界性质是使用Gibbs合奏的连续分数分量版本通过Monte Carlo模拟获得的。饱和蒸气压是从NPT模拟获得的。在仿真结果与文献数据(使用Ewald求和的仿真)之间发现了极好的一致性。还显示了如何为Wolf方法选择最佳参数。尽管沃尔夫方法需要在气相中使用大型仿真箱,但由于缺少静电屏蔽,因此人们可以将沃尔夫方法视为VLE计算中Ewald求和的一种合适替代方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号