当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
KRAS Switch Mutants D33E and A59G Crystallize in the State 1 Conformation
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-28 00:00:00 , DOI: 10.1021/acs.biochem.7b00974 Jia Lu 1 , Asim K Bera 1 , Sudershan Gondi 1 , Kenneth D Westover 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-28 00:00:00 , DOI: 10.1021/acs.biochem.7b00974 Jia Lu 1 , Asim K Bera 1 , Sudershan Gondi 1 , Kenneth D Westover 1
Affiliation
|
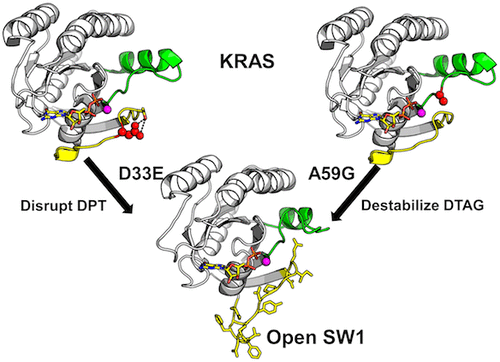
KRAS switch loop movements play a crucial role in regulating RAS signaling, and alteration of these sensitive dynamics is a principal mechanism through which disease-associated RAS mutations lead to aberrant RAS activation. Prior studies suggest that despite a high degree of sequence similarity, the switches in KRAS are more dynamic than those in HRAS. We determined X-ray crystal structures of the rare tumorigenic KRAS mutants KRASD33E, in switch 1 (SW1), and KRASA59G, in switch 2 (SW2), bound to GDP and found these adopt nearly identical, open SW1 conformations as well as altered SW2 conformations. KRASA59G bound to a GTP analogue crystallizes in the same conformation. This open conformation is consistent with the inactive “state 1” previously observed for HRAS bound to GTP. For KRASA59G, switch rearrangements may be regulated by increased flexibility in the 57DXXGQ61 motif at codon 59. However, loss of interactions between side chains at codons 33 and 35 in the SW1 33DPT35 motif drives changes for KRASD33E. The 33DPT35 motif is conserved for multiple members of the RAS subfamily but is not found in RAB, RHO, ARF, or Gα families, suggesting that dynamics mediated by this motif may be important for determining the selectivity of RAS–effector interactions. Biochemically, the consequence of altered switch dynamics is the same, showing impaired interaction with the guanine exchange factor SOS and loss of GAP-dependent GTPase activity. However, interactions with the RBD of RAF are preserved. Overall, these observations add to a body of evidence suggesting that HRAS and KRAS show meaningful differences in functionality stemming from differential protein dynamics independent of the hypervariable region.
中文翻译:

KRAS 开关突变体 D33E 和 A59G 在状态 1 构象中结晶
KRAS 开关回路运动在调节 RAS 信号传导中起着至关重要的作用,这些敏感动力学的改变是疾病相关 RAS 突变导致异常 RAS 激活的主要机制。先前的研究表明,尽管具有高度的序列相似性,但 KRAS 中的开关比 HRAS 中的开关更具动态性。我们确定了开关 1 (SW1)中的罕见致瘤 KRAS 突变体 KRAS D33E和开关 2 (SW2) 中与 GDP 结合的KRAS A59G 的X 射线晶体结构,发现它们采用几乎相同的开放 SW1 构象以及改变了 SW2 构象。KRAS A59G绑定到 GTP 类似物结晶在相同的构象。这种开放构象与先前观察到的与 GTP 结合的 HRAS 的非活动“状态 1”一致。对于 KRAS A59G,开关重排可以通过密码子 59 处57 DXXGQ 61基序的灵活性增加来调节。然而,SW1 33 DPT 35基序中密码子 33 和 35 侧链之间相互作用的丧失驱动了 KRAS D33E 的变化。在33 DPT 35基序对于 RAS 亚家族的多个成员是保守的,但在 RAB、RHO、ARF 或 Gα 家族中未发现,这表明由该基序介导的动力学可能对于确定 RAS 效应子相互作用的选择性很重要。在生化方面,改变开关动力学的结果是相同的,显示与鸟嘌呤交换因子 SOS 的相互作用受损和依赖 GAP 的 GTPase 活性丧失。但是,保留了与 RAF 的 RBD 的相互作用。总体而言,这些观察结果补充了大量证据,表明 HRAS 和 KRAS 在功能上显示出有意义的差异,这些差异源于独立于高变区的差异蛋白质动力学。
更新日期:2017-12-28
中文翻译:

KRAS 开关突变体 D33E 和 A59G 在状态 1 构象中结晶
KRAS 开关回路运动在调节 RAS 信号传导中起着至关重要的作用,这些敏感动力学的改变是疾病相关 RAS 突变导致异常 RAS 激活的主要机制。先前的研究表明,尽管具有高度的序列相似性,但 KRAS 中的开关比 HRAS 中的开关更具动态性。我们确定了开关 1 (SW1)中的罕见致瘤 KRAS 突变体 KRAS D33E和开关 2 (SW2) 中与 GDP 结合的KRAS A59G 的X 射线晶体结构,发现它们采用几乎相同的开放 SW1 构象以及改变了 SW2 构象。KRAS A59G绑定到 GTP 类似物结晶在相同的构象。这种开放构象与先前观察到的与 GTP 结合的 HRAS 的非活动“状态 1”一致。对于 KRAS A59G,开关重排可以通过密码子 59 处57 DXXGQ 61基序的灵活性增加来调节。然而,SW1 33 DPT 35基序中密码子 33 和 35 侧链之间相互作用的丧失驱动了 KRAS D33E 的变化。在33 DPT 35基序对于 RAS 亚家族的多个成员是保守的,但在 RAB、RHO、ARF 或 Gα 家族中未发现,这表明由该基序介导的动力学可能对于确定 RAS 效应子相互作用的选择性很重要。在生化方面,改变开关动力学的结果是相同的,显示与鸟嘌呤交换因子 SOS 的相互作用受损和依赖 GAP 的 GTPase 活性丧失。但是,保留了与 RAF 的 RBD 的相互作用。总体而言,这些观察结果补充了大量证据,表明 HRAS 和 KRAS 在功能上显示出有意义的差异,这些差异源于独立于高变区的差异蛋白质动力学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号