当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of Allylic Fluorides via Fluorination of Racemic Allylic Trichloroacetimidates Catalyzed by a Chiral Diene-Iridium Complex
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-12-29 00:00:00 , DOI: 10.1021/acscatal.7b03786 Jason C. Mixdorf 1 , Alexandre M. Sorlin 1 , Qi Zhang 1 , Hien M. Nguyen 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-12-29 00:00:00 , DOI: 10.1021/acscatal.7b03786 Jason C. Mixdorf 1 , Alexandre M. Sorlin 1 , Qi Zhang 1 , Hien M. Nguyen 1
Affiliation
|
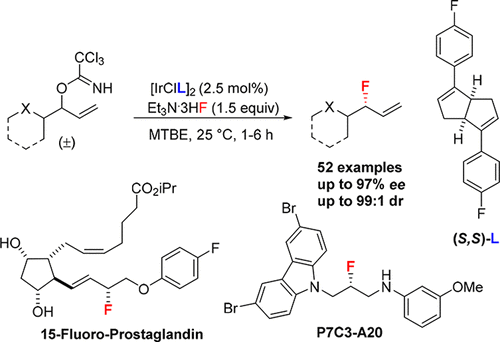
The ability to use racemic allylic trichloroacetimidates as competent electrophiles in a chiral bicyclo[3.3.0]octadiene-ligated iridium-catalyzed asymmetric fluorination with Et3N·3HF is described. The methodology represents an effective route to prepare a wide variety of α-linear, α-branching, and β-heteroatom substituted allylic fluorides in good yields, excellent branched-to-linear ratios, and high levels of enantioselectivity. Additionally, the catalytic system is amendable to the fluorination of optically active allylic trichloroacetimidate substrates to afford the fluorinated products in good yields with exclusively branched selectivity. Excellent levels of catalyst-controlled diastereoselectivities using either (R,R) or (S,S)-bicyclo[3.3.0]octadiene ligand are observed. The synthetic utility of the fluorination process is illustrated in the asymmetric synthesis of 15-fluorinated prostaglandin and neuroprotective agent P7C3-A20.
中文翻译:

手性二烯-铱配合物催化外消旋烯丙基三氯乙亚胺酸酯的氟化不对称合成烯丙基氟
描述了在Et 3 N·3HF手性双环[3.3.0]辛二烯连接的铱催化的不对称氟化反应中使用外消旋烯丙基三氯乙亚氨酸盐作为感受态亲电试剂的能力。该方法代表了一种以高收率,出色的支化-线性比率和高对映选择性制备各种α-线性,α-支化和β-杂原子取代的烯丙基氟化物的有效途径。另外,该催化体系可修正光学活性的烯丙基三氯乙酰亚氨酸酯底物的氟化,以高收率提供氟化产物,并且具有专门的支链选择性。使用(R,R)或(S,观察到S)-双环[3.3.0]辛二烯配体。氟化过程的合成用途在15氟化前列腺素和神经保护剂P7C3-A20的不对称合成中得到了说明。
更新日期:2017-12-29
中文翻译:

手性二烯-铱配合物催化外消旋烯丙基三氯乙亚胺酸酯的氟化不对称合成烯丙基氟
描述了在Et 3 N·3HF手性双环[3.3.0]辛二烯连接的铱催化的不对称氟化反应中使用外消旋烯丙基三氯乙亚氨酸盐作为感受态亲电试剂的能力。该方法代表了一种以高收率,出色的支化-线性比率和高对映选择性制备各种α-线性,α-支化和β-杂原子取代的烯丙基氟化物的有效途径。另外,该催化体系可修正光学活性的烯丙基三氯乙酰亚氨酸酯底物的氟化,以高收率提供氟化产物,并且具有专门的支链选择性。使用(R,R)或(S,观察到S)-双环[3.3.0]辛二烯配体。氟化过程的合成用途在15氟化前列腺素和神经保护剂P7C3-A20的不对称合成中得到了说明。











































 京公网安备 11010802027423号
京公网安备 11010802027423号