当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Immunoglobulin Fc-Fused Peptide without C-Terminal Arg or Lys Residue Augments Neuropilin-1-Dependent Tumor Vascular Permeability
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-12-21 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00761 Du-San Baek 1 , Jeong-Ho Kim 1 , Ye-Jin Kim 1 , Yong-Sung Kim 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-12-21 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00761 Du-San Baek 1 , Jeong-Ho Kim 1 , Ye-Jin Kim 1 , Yong-Sung Kim 1
Affiliation
|
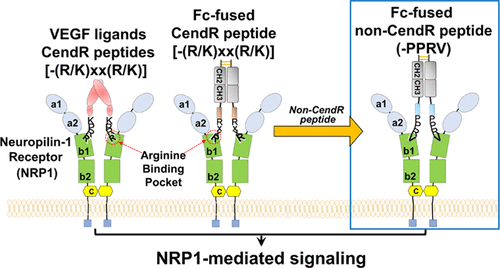
Neuropilin-1 (NRP1), which functions as a coreceptor for vascular endothelial growth factor (VEGF) and is implicated in vascular permeability and tumorigenesis, has been targeted by peptides that specifically bind to the VEGF-binding region on NRP1. Like natural VEGF ligands, all known peptides with NRP1-binding activity bind only through a carboxy (C)-terminal R/K-x-x-R/K sequence motif (x stands for any amino acids); this strict requirement is called the C-end rule (CendR). Here, we report immunoglobulin Fc-fused NRP1-specific peptides deviating from CendR. We screened a yeast surface-displayed Fc-fused non-CendR peptide library against NRP1 and isolated Fc-V12, wherein V12 peptide comprising 12 amino acids has a PPRV sequence at its C-terminal end. Although Fc-V12 lacked the CendR motif, it showed selective binding to the VEGF-binding region of NRP1 and triggered cellular internalization of NRP1, which resulted in enhanced extravasation into tumor tissues and tumor tissue penetration of the Fc-fused peptide along with the coinjected chemical drug in tumor-bearing mice. Through a saturation mutagenesis study, we identified that the Val residue at the C-terminus of Fc-V12 is crucial for NRP1 binding. We further improved NRP1 affinity of Fc-V12 (KD = ∼761 nM) through directed evolution of the upstream sequence of PPRV to obtain Fc-V12–33 (KD = ∼17.4 nM), which exhibited enhanced NRP1-mediated vascular permeability as compared with Fc-V12. Our results provide functional Fc-fused non-CendR peptides, which bind to the VEGF-binding region of NRP1 and enhance vascular permeability, expanding the sequence space of NRP1-targeting peptides.
中文翻译:

无C末端Arg或Lys残基增强的免疫球蛋白Fc融合肽Neuropilin-1依赖性肿瘤血管通透性
Neuropilin-1(NRP1)作为血管内皮生长因子(VEGF)的共受体,与血管通透性和肿瘤发生有关,已被特异性结合NRP1上VEGF结合区的肽所靶向。像天然VEGF配体一样,所有具有NRP1结合活性的已知肽仅通过羧基(C)末端的R / KxxR / K序列基序结合(x代表任何氨基酸);此严格要求称为C端规则(CendR)。在这里,我们报告了偏离CendR的免疫球蛋白Fc融合NRP1特异性肽段。我们筛选了针对NRP1和分离的Fc-V12的酵母表面展示的Fc融合非CendR肽文库,其中包含12个氨基酸的V12肽在其C末端具有PPRV序列。尽管Fc-V12缺乏CendR基序,它显示出与NRP1的VEGF结合区的选择性结合,并触发了NRP1的细胞内在化,从而导致了向肿瘤组织的外渗和Fc融合肽以及共同注射的化学药物在荷瘤小鼠中的肿瘤组织渗透的增强。通过饱和诱变研究,我们确定了Fc-V12 C端的Val残基对于NRP1结合至关重要。我们进一步提高了Fc-V12的NRP1亲和力(K D =〜761 nM),是通过PPRV上游序列的定向进化获得的Fc-V12-33(K D =〜17.4 nM),与Fc-V12相比,NRP1介导的血管通透性增强。我们的结果提供了功能性Fc融合的非CendR肽,该肽与NRP1的VEGF结合区结合并增强血管通透性,从而扩大了靶向NRP1的肽的序列空间。
更新日期:2017-12-21
中文翻译:

无C末端Arg或Lys残基增强的免疫球蛋白Fc融合肽Neuropilin-1依赖性肿瘤血管通透性
Neuropilin-1(NRP1)作为血管内皮生长因子(VEGF)的共受体,与血管通透性和肿瘤发生有关,已被特异性结合NRP1上VEGF结合区的肽所靶向。像天然VEGF配体一样,所有具有NRP1结合活性的已知肽仅通过羧基(C)末端的R / KxxR / K序列基序结合(x代表任何氨基酸);此严格要求称为C端规则(CendR)。在这里,我们报告了偏离CendR的免疫球蛋白Fc融合NRP1特异性肽段。我们筛选了针对NRP1和分离的Fc-V12的酵母表面展示的Fc融合非CendR肽文库,其中包含12个氨基酸的V12肽在其C末端具有PPRV序列。尽管Fc-V12缺乏CendR基序,它显示出与NRP1的VEGF结合区的选择性结合,并触发了NRP1的细胞内在化,从而导致了向肿瘤组织的外渗和Fc融合肽以及共同注射的化学药物在荷瘤小鼠中的肿瘤组织渗透的增强。通过饱和诱变研究,我们确定了Fc-V12 C端的Val残基对于NRP1结合至关重要。我们进一步提高了Fc-V12的NRP1亲和力(K D =〜761 nM),是通过PPRV上游序列的定向进化获得的Fc-V12-33(K D =〜17.4 nM),与Fc-V12相比,NRP1介导的血管通透性增强。我们的结果提供了功能性Fc融合的非CendR肽,该肽与NRP1的VEGF结合区结合并增强血管通透性,从而扩大了靶向NRP1的肽的序列空间。











































 京公网安备 11010802027423号
京公网安备 11010802027423号