当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamic Strategic Bond Analysis Yields a Ten-Step Synthesis of 20-nor-Salvinorin A, a Potent κ-OR Agonist
ACS Central Science ( IF 12.7 ) Pub Date : 2017-12-13 00:00:00 , DOI: 10.1021/acscentsci.7b00488 Jeremy J. Roach 1 , Yusuke Sasano 1 , Cullen L. Schmid 2 , Saheem Zaidi 3 , Vsevolod Katritch 3 , Raymond C. Stevens 3 , Laura M. Bohn 2 , Ryan A. Shenvi 1
ACS Central Science ( IF 12.7 ) Pub Date : 2017-12-13 00:00:00 , DOI: 10.1021/acscentsci.7b00488 Jeremy J. Roach 1 , Yusuke Sasano 1 , Cullen L. Schmid 2 , Saheem Zaidi 3 , Vsevolod Katritch 3 , Raymond C. Stevens 3 , Laura M. Bohn 2 , Ryan A. Shenvi 1
Affiliation
|
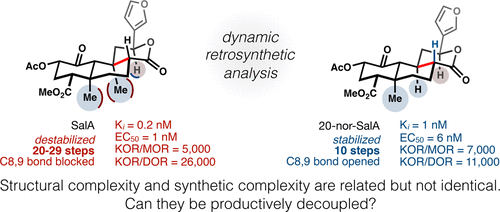
Salvinorin A (SalA) is a plant metabolite that agonizes the human kappa-opioid receptor (κ-OR) with high affinity and high selectivity over mu- and delta-opioid receptors. Its therapeutic potential has stimulated extensive semisynthetic studies and total synthesis campaigns. However, structural modification of SalA has been complicated by its instability, and efficient total synthesis has been frustrated by its dense, complex architecture. Treatment of strategic bonds in SalA as dynamic and dependent on structural perturbation enabled the identification of an efficient retrosynthetic pathway. Here we show that deletion of C20 simultaneously stabilizes the SalA skeleton, simplifies its synthesis, and retains its high affinity and selectivity for the κ-OR. The resulting 10-step synthesis now opens the SalA scaffold to deep-seated property modification. Finally, we describe a workflow to identify structural changes that retain molecular complexity, but reduce synthetic complexity—two related, but independent ways of looking at complexity.
中文翻译:

动态战略债券分析产生了有效的κ-OR激动剂20-nor-Salvinorin A的十步合成方法。
Salvinorin A(SalA)是一种植物代谢产物,它对人类Kappa类阿片受体(κ-OR)具有较高的亲和力,对mu-和delta-的选择性高阿片受体。它的治疗潜力激发了广泛的半合成研究和全合成运动。但是,SalA的结构修饰因其不稳定性而变得复杂,而有效的全合成却因其致密,复杂的结构而受挫。SalA中策略键的动态处理以及依赖于结构微扰的处理使得能够确定有效的逆合成途径。在这里,我们表明删除C20同时稳定了SalA骨架,简化了其合成,并保留了其对κ-OR的高亲和力和选择性。现在,最终的10步合成使SalA支架可以进行深层次的特性修饰。最后,我们描述一种工作流程,以识别保留分子复杂性但降低合成复杂性的结构变化-两个相关的,
更新日期:2017-12-13
中文翻译:

动态战略债券分析产生了有效的κ-OR激动剂20-nor-Salvinorin A的十步合成方法。
Salvinorin A(SalA)是一种植物代谢产物,它对人类Kappa类阿片受体(κ-OR)具有较高的亲和力,对mu-和delta-的选择性高阿片受体。它的治疗潜力激发了广泛的半合成研究和全合成运动。但是,SalA的结构修饰因其不稳定性而变得复杂,而有效的全合成却因其致密,复杂的结构而受挫。SalA中策略键的动态处理以及依赖于结构微扰的处理使得能够确定有效的逆合成途径。在这里,我们表明删除C20同时稳定了SalA骨架,简化了其合成,并保留了其对κ-OR的高亲和力和选择性。现在,最终的10步合成使SalA支架可以进行深层次的特性修饰。最后,我们描述一种工作流程,以识别保留分子复杂性但降低合成复杂性的结构变化-两个相关的,











































 京公网安备 11010802027423号
京公网安备 11010802027423号