当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Novel Selective Acetyl-CoA Carboxylase (ACC) 1 Inhibitors
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01547 Ryo Mizojiri 1 , Moriteru Asano 1 , Daisuke Tomita 1 , Hiroshi Banno 1 , Noriyuki Nii 1 , Masako Sasaki 1 , Hiroyuki Sumi 1 , Yoshihiko Satoh 1 , Yukiko Yamamoto 1 , Takeo Moriya 1 , Yoshinori Satomi 1 , Hironobu Maezaki 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01547 Ryo Mizojiri 1 , Moriteru Asano 1 , Daisuke Tomita 1 , Hiroshi Banno 1 , Noriyuki Nii 1 , Masako Sasaki 1 , Hiroyuki Sumi 1 , Yoshihiko Satoh 1 , Yukiko Yamamoto 1 , Takeo Moriya 1 , Yoshinori Satomi 1 , Hironobu Maezaki 1
Affiliation
|
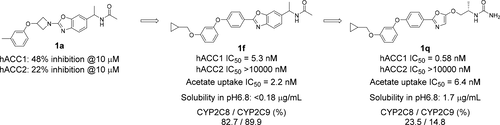
We initiated our structure–activity relationship (SAR) studies for selective ACC1 inhibitors from 1a as a lead compound. SAR studies of bicyclic scaffolds revealed many potent and selective ACC1 inhibitors represented by 1f; however most of them had physicochemical issues, particularly low aqueous solubility and potent CYP inhibition. To address these two issues and improve the druglikeness of this chemical series, we converted the bicyclic scaffold into a monocyclic framework. Ultimately, this lead us to discover a novel monocyclic derivative 1q as a selective ACC1 inhibitor, which showed highly potent and selective ACC1 inhibition as well as acceptable solubility and CYP inhibition profiles. Since compound 1q displayed favorable bioavailability in mouse cassette dosing testing, we conducted in vivo PD studies of this compound. Oral administration of 1q significantly reduced the concentration of malonyl-CoA in HCT-116 xenograft tumors at doses of more than 30 mg/kg. Accordingly, our novel series of selective ACC1 inhibitors represents a set of useful orally available research tools, as well as potential therapeutic agents for cancer and fatty acid related diseases.
中文翻译:

新型选择性乙酰辅酶A羧化酶(ACC)1抑制剂的发现。
我们开始了对以1a为主要化合物的选择性ACC1抑制剂的结构-活性关系(SAR)研究。SAR研究的双环支架显示许多有效的和选择性的ACC1抑制剂以1f代表; 但是它们中的大多数具有理化问题,特别是水溶性低和有效的CYP抑制作用。为了解决这两个问题并改善该化学系列的药物似性,我们将双环支架转换为单环框架。最终,这使我们发现了一种新型的单环衍生物1q作为选择性ACC1抑制剂,它表现出强效和选择性的ACC1抑制作用以及可接受的溶解度和CYP抑制作用。自化合物1q在小鼠盒式剂量测试中显示出良好的生物利用度,因此我们对该化合物进行了体内PD研究。口服1q剂量大于30 mg / kg时,可显着降低HCT-116异种移植肿瘤中丙二酰辅酶A的浓度。因此,我们的新型选择性ACC1抑制剂系列代表了一组有用的口服研究工具,以及癌症和脂肪酸相关疾病的潜在治疗剂。
更新日期:2018-01-05
中文翻译:

新型选择性乙酰辅酶A羧化酶(ACC)1抑制剂的发现。
我们开始了对以1a为主要化合物的选择性ACC1抑制剂的结构-活性关系(SAR)研究。SAR研究的双环支架显示许多有效的和选择性的ACC1抑制剂以1f代表; 但是它们中的大多数具有理化问题,特别是水溶性低和有效的CYP抑制作用。为了解决这两个问题并改善该化学系列的药物似性,我们将双环支架转换为单环框架。最终,这使我们发现了一种新型的单环衍生物1q作为选择性ACC1抑制剂,它表现出强效和选择性的ACC1抑制作用以及可接受的溶解度和CYP抑制作用。自化合物1q在小鼠盒式剂量测试中显示出良好的生物利用度,因此我们对该化合物进行了体内PD研究。口服1q剂量大于30 mg / kg时,可显着降低HCT-116异种移植肿瘤中丙二酰辅酶A的浓度。因此,我们的新型选择性ACC1抑制剂系列代表了一组有用的口服研究工具,以及癌症和脂肪酸相关疾病的潜在治疗剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号