Advanced Drug Delivery Reviews ( IF 15.2 ) Pub Date : 2017-12-12 , DOI: 10.1016/j.addr.2017.12.002 Jay M Newby 1 , Ian Seim 1 , Martin Lysy 2 , Yun Ling 2 , Justin Huckaby 3 , Samuel K Lai 4 , M Gregory Forest 5
|
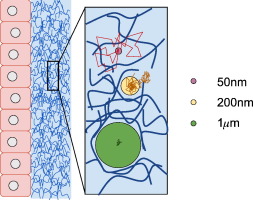
In mucosal drug delivery, two design goals are desirable: 1) insure drug passage through the mucosal barrier to the epithelium prior to drug removal from the respective organ via mucus clearance; and 2) design carrier particles to achieve a prescribed arrival time and drug uptake schedule at the epithelium. Both goals are achievable if one can control “one-sided” diffusive passage times of drug carrier particles: from deposition at the mucus interface, through the mucosal barrier, to the epithelium. The passage time distribution must be, with high confidence, shorter than the timescales of mucus clearance to maximize drug uptake. For 100 nm and smaller drug-loaded nanoparticulates, as well as pure drug powders or drug solutions, diffusion is normal (i.e., Brownian) and rapid, easily passing through the mucosal barrier prior to clearance. Major challenges in quantitative control over mucosal drug delivery lie with larger drug-loaded nanoparticulates that are comparable to or larger than the pores within the mucus gel network, for which diffusion is not simple Brownian motion and typically much less rapid; in these scenarios, a timescale competition ensues between particle passage through the mucus barrier and mucus clearance from the organ. In the lung, as a primary example, coordinated cilia and air drag continuously transport mucus toward the trachea, where mucus and trapped cargo are swallowed into the digestive tract. Mucus clearance times in lung airways range from minutes to hours or significantly longer depending on deposition in the upper, middle, lower airways and on lung health, giving a wide time window for drug-loaded particle design to achieve controlled delivery to the epithelium. We review the physical and chemical factors (of both particles and mucus) that dictate particle diffusion in mucus, and the technological strategies (theoretical and experimental) required to achieve the design goals. First we describe an idealized scenario — a homogeneous viscous fluid of uniform depth with a particle undergoing passive normal diffusion — where the theory of Brownian motion affords the ability to rigorously specify particle size distributions to meet a prescribed, one-sided, diffusive passage time distribution. Furthermore, we describe how the theory of Brownian motion provides the scaling of one-sided diffusive passage times with respect to mucus viscosity and layer depth, and under reasonable caveats, one can also prescribe passage time scaling due to heterogeneity in viscosity and layer depth. Small-molecule drugs and muco-inert, drug-loaded carrier particles 100 nm and smaller fall into this class of rigorously controllable passage times for drug delivery. Second we describe the prevalent scenarios in which drug-loaded carrier particles in mucus violate simple Brownian motion, instead exhibiting anomalous sub-diffusion, for which all theoretical control over diffusive passage times is lost, and experiments are prohibitive if not impossible to measure one-sided passage times. We then discuss strategies to overcome these roadblocks, requiring new particle-tracking experiments and emerging advances in theory and computation of anomalous, sub-diffusive processes that are necessary to predict and control one-sided particle passage times from deposition at the mucosal interface to epithelial uptake. We highlight progress to date, remaining hurdles, and prospects for achieving the two design goals for 200 nm and larger, drug-loaded, non-dissolving, nanoparticulates.
中文翻译:

估计和控制粘膜药物输送中穿过粘液屏障的扩散通过时间的技术策略
在粘膜药物递送中,需要两个设计目标:1)确保药物在通过粘液清除从相应器官中去除之前穿过粘膜屏障到达上皮; 2) 设计载体颗粒以达到上皮细胞规定的到达时间和药物摄取时间表。如果能够控制药物载体颗粒的“单侧”扩散通过时间:从粘液界面沉积,穿过粘膜屏障,到达上皮,这两个目标都是可以实现的。通过时间分布必须以高置信度短于粘液清除的时间尺度,以最大限度地提高药物吸收。对于100 nm和更小的载药纳米颗粒,以及纯药物粉末或药物溶液,扩散是正常的(即布朗)并且快速,在清除之前很容易穿过粘膜屏障。粘膜药物递送定量控制的主要挑战在于较大的载药纳米颗粒,其与粘液凝胶网络内的孔相当或更大,其扩散不是简单的布朗运动,并且通常速度慢得多;在这些情况下,粒子通过粘液屏障和粘液从器官清除之间会发生时间尺度的竞争。以肺部为例,协调的纤毛和空气阻力不断地将粘液输送到气管,粘液和滞留的物质被吞入消化道。肺气道中的粘液清除时间从几分钟到几小时不等,甚至更长,具体取决于上、中、下气道的沉积和肺部健康状况,这为载药颗粒设计提供了较宽的时间窗口,以实现对上皮的控制递送。 我们回顾了决定粘液中颗粒扩散的物理和化学因素(颗粒和粘液),以及实现设计目标所需的技术策略(理论和实验)。首先,我们描述一个理想化的场景 - 具有均匀深度的均质粘性流体,其中粒子经历被动法向扩散 - 其中布朗运动理论提供了严格指定粒子尺寸分布的能力,以满足规定的、单侧的、扩散的通过时间分布。此外,我们描述了布朗运动理论如何提供相对于粘液粘度和层深度的一侧扩散通过时间的标度,并且在合理的警告下,由于粘度和层深度的不均匀性,人们还可以规定通过时间标度。小分子药物和粘液惰性、载药载体颗粒(100 nm 或更小)属于此类严格可控的药物输送通过时间。其次,我们描述了常见的情况,其中粘液中的载药载体颗粒违反简单的布朗运动,而是表现出异常的子扩散,为此,对扩散通过时间的所有理论控制都丢失了,并且实验即使不是不可能测量一个,也是令人望而却步的。侧面通过时间。然后,我们讨论克服这些障碍的策略,需要新的粒子跟踪实验以及异常、亚扩散过程的理论和计算方面的新兴进展,这些进展对于预测和控制从粘膜界面沉积到上皮的单侧粒子通过时间是必要的吸收。 我们重点介绍了迄今为止取得的进展、剩余的障碍以及实现 200 nm 及更大的载药、不溶解纳米颗粒的两个设计目标的前景。











































 京公网安备 11010802027423号
京公网安备 11010802027423号