当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Studies on Tryptophan Lyase (NosL): Identification of Cyanide as a Reaction Product
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-01-02 , DOI: 10.1021/jacs.7b09000 Dhananjay M Bhandari 1 , Dmytro Fedoseyenko 1 , Tadhg P Begley 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-01-02 , DOI: 10.1021/jacs.7b09000 Dhananjay M Bhandari 1 , Dmytro Fedoseyenko 1 , Tadhg P Begley 1
Affiliation
|
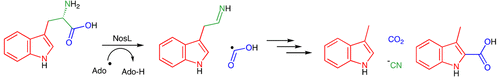
Tryptophan lyase (NosL) catalyzes the formation of 3-methylindole-2-carboxylic acid and 3-methylindole from l-tryptophan. In this paper, we provide evidence supporting a formate radical intermediate and demonstrate that cyanide is a byproduct of the NosL-catalyzed reaction with l-tryptophan. These experiments require a major revision of the NosL mechanism and uncover an unanticipated connection between NosL and HydG, the radical SAM enzyme that forms cyanide and carbon monoxide from tyrosine during the biosynthesis of the metallo-cluster of the [Fe-Fe] hydrogenase.
中文翻译:

色氨酸裂解酶 (NosL) 的机理研究:反应产物氰化物的鉴定
色氨酸裂解酶 (NosL) 催化 L-色氨酸形成 3-甲基吲哚-2-羧酸和 3-甲基吲哚。在本文中,我们提供了支持甲酸盐自由基中间体的证据,并证明氰化物是 NosL 催化的 L-色氨酸反应的副产物。这些实验需要对 NosL 机制进行重大修改,并揭示 NosL 和 HydG 之间意想不到的联系,HydG 是一种自由基 SAM 酶,在 [Fe-Fe] 氢化酶金属簇的生物合成过程中从酪氨酸形成氰化物和一氧化碳。
更新日期:2018-01-02
中文翻译:

色氨酸裂解酶 (NosL) 的机理研究:反应产物氰化物的鉴定
色氨酸裂解酶 (NosL) 催化 L-色氨酸形成 3-甲基吲哚-2-羧酸和 3-甲基吲哚。在本文中,我们提供了支持甲酸盐自由基中间体的证据,并证明氰化物是 NosL 催化的 L-色氨酸反应的副产物。这些实验需要对 NosL 机制进行重大修改,并揭示 NosL 和 HydG 之间意想不到的联系,HydG 是一种自由基 SAM 酶,在 [Fe-Fe] 氢化酶金属簇的生物合成过程中从酪氨酸形成氰化物和一氧化碳。











































 京公网安备 11010802027423号
京公网安备 11010802027423号