当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective C−H Halogenation with a Highly Fluorinated Manganese Porphyrin
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-12-28 , DOI: 10.1002/anie.201710676 Gang Li 1 , Andrew K. Dilger 2 , Peter T. Cheng 2 , William R. Ewing 2 , John T. Groves 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-12-28 , DOI: 10.1002/anie.201710676 Gang Li 1 , Andrew K. Dilger 2 , Peter T. Cheng 2 , William R. Ewing 2 , John T. Groves 1
Affiliation
|
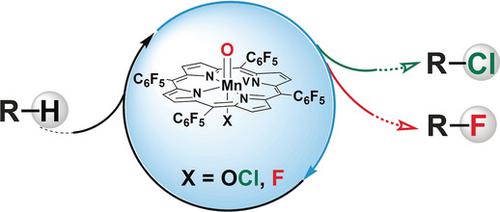
The selective C−H functionalization of aliphatic molecules remains a challenge in organic synthesis. While radical chain halogenation reactions provide efficient access to many halogenated molecules, the use of typical protocols for the selective halogenation of electron‐deficient and strained aliphatic molecules is rare. Herein, we report selective C−H chlorination and fluorination reactions promoted by an electron‐deficient manganese pentafluorophenyl porphyrin catalyst, Mn(TPFPP)Cl. This catalyst displays superior properties for the aliphatic halogenation of recalcitrant, electron‐deficient, and strained substrates with unique regio‐ and stereoselectivity. UV/Vis analysis during the course of the reaction indicated that an oxo‐MnV species is responsible for hydrogen‐atom abstraction. The observed stereoselectivity results from steric interactions between the bulky porphyrin ligand and the intermediate substrate radical in the halogen rebound step.
中文翻译:

用高度氟化的锰卟啉进行选择性CH卤化
脂肪族分子的选择性C H功能化仍然是有机合成中的一个挑战。尽管自由基链卤化反应可以有效地接近许多卤化分子,但很少使用典型的方案对电子缺乏和应变的脂族分子进行选择性卤化。在本文中,我们报告了由缺电子的五氟苯基锰卟啉催化剂Mn(TPFPP)Cl促进的选择性CH氯化和氟化反应。该催化剂对难分解的,电子缺陷的和应变的底物的脂族卤代具有独特的区域和立体选择性,显示出优异的性能。反应过程中的UV / Vis分析表明,氧-Mn V物种负责氢原子的提取。所观察到的立体选择性是由于大体积的卟啉配体与中间底物自由基在卤素回弹步骤中的空间相互作用而产生的。
更新日期:2017-12-28
中文翻译:

用高度氟化的锰卟啉进行选择性CH卤化
脂肪族分子的选择性C H功能化仍然是有机合成中的一个挑战。尽管自由基链卤化反应可以有效地接近许多卤化分子,但很少使用典型的方案对电子缺乏和应变的脂族分子进行选择性卤化。在本文中,我们报告了由缺电子的五氟苯基锰卟啉催化剂Mn(TPFPP)Cl促进的选择性CH氯化和氟化反应。该催化剂对难分解的,电子缺陷的和应变的底物的脂族卤代具有独特的区域和立体选择性,显示出优异的性能。反应过程中的UV / Vis分析表明,氧-Mn V物种负责氢原子的提取。所观察到的立体选择性是由于大体积的卟啉配体与中间底物自由基在卤素回弹步骤中的空间相互作用而产生的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号