当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Kinetic Dearomatization Strategy for an Expedient Biomimetic Route to the Bielschowskysin Skeleton.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-05 , DOI: 10.1002/anie.201711780 Paul Scesa 1 , Medhi Wangpaichitr 2 , Niramol Savaraj 2 , Lyndon West 1 , Stéphane P Roche 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-01-05 , DOI: 10.1002/anie.201711780 Paul Scesa 1 , Medhi Wangpaichitr 2 , Niramol Savaraj 2 , Lyndon West 1 , Stéphane P Roche 1
Affiliation
|
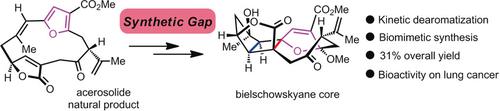
Bielschowskysin (1), the flagship of the furanocembranoid diterpene family, has attracted attention from chemists owing to its intriguing and daunting polycyclic architecture and medicinal potential against lung cancer. The high level of functionalization of 1 poses a considerable challenge to synthesis. Herein, a stereoselective furan dearomatization strategy of furanocembranoids was achieved via the intermediacy of chlorohydrins. The stereochemical course of the kinetic dearomatization was established, and the C3 configuration of the resulting exo‐enol ether intermediates proved to be essential to complete the late‐stage transannular [2+2] photocycloaddition. Overall, this biomimetic strategy starting from the natural product acerosolide (9) featured an unprecedented regio‐ and highly stereoselective furan dearomatization, which provided rapid access to the pivotal exo‐enol ethers en route to the intricate bielschowskyane skeleton.
中文翻译:

一种动力学脱芳香化策略,可方便地仿制Bielschowskysin骨架。
呋喃西兰二萜家族的旗舰产品Bielschowskysin(1)由于其有趣的,令人生畏的多环结构以及对肺癌的药用潜力而引起了化学家的关注。功能化的高级别1构成,以合成一个相当大的挑战。在此,通过氯代醇的中间产物实现了呋喃类呋喃类化合物的立体选择性呋喃脱芳香化策略。建立了动力学脱芳香化反应的立体化学过程,并证明了所得到的外-烯醇醚中间体的C3构型对于完成后期跨环[2 + 2]光环加成至关重要。总体而言,这种仿生策略始于天然产物acerosolide(9)具有前所未有的区域和高度立体选择性的呋喃脱芳香化作用,可在进入复杂的Bielschowskyane骨架的过程中快速进入关键的外-烯醇。
更新日期:2018-01-05
中文翻译:

一种动力学脱芳香化策略,可方便地仿制Bielschowskysin骨架。
呋喃西兰二萜家族的旗舰产品Bielschowskysin(1)由于其有趣的,令人生畏的多环结构以及对肺癌的药用潜力而引起了化学家的关注。功能化的高级别1构成,以合成一个相当大的挑战。在此,通过氯代醇的中间产物实现了呋喃类呋喃类化合物的立体选择性呋喃脱芳香化策略。建立了动力学脱芳香化反应的立体化学过程,并证明了所得到的外-烯醇醚中间体的C3构型对于完成后期跨环[2 + 2]光环加成至关重要。总体而言,这种仿生策略始于天然产物acerosolide(9)具有前所未有的区域和高度立体选择性的呋喃脱芳香化作用,可在进入复杂的Bielschowskyane骨架的过程中快速进入关键的外-烯醇。











































 京公网安备 11010802027423号
京公网安备 11010802027423号