当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic Asymmetric Synthesis of Trifluoromethylated γ‐Amino Acids through the Umpolung Addition of Trifluoromethyl Imines to Carboxylic Acid Derivatives
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-01-18 , DOI: 10.1002/anie.201710915 Bin Hu 1 , Li Deng 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-01-18 , DOI: 10.1002/anie.201710915 Bin Hu 1 , Li Deng 1
Affiliation
|
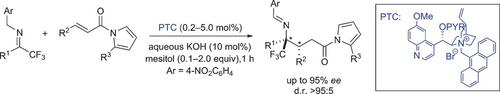
Novel cinchona alkaloid derived chiral phase‐transfer catalysts enabled the highly chemo‐, regio‐, diastereo‐, and enantioselective umpolung addition of trifluoromethyl imines to α,β‐unsaturated N‐acyl pyrroles. With a catalyst loading ranging from 0.2 to 5.0 mol %, this new catalytic asymmetric transformation provides facile and high‐yielding access to highly enantiomerically enriched chiral trifluoromethylated γ‐amino acids and γ‐lactams.
中文翻译:

通过三氟甲基亚胺与羧酸衍生物的 Umpolung 加成催化不对称合成三氟甲基化 γ-氨基酸
新型金鸡纳生物碱衍生的手性相转移催化剂能够实现三氟甲基亚胺与α,β-不饱和N-酰基吡咯的高度化学、区域、非对映和对映选择性反极性加成。这种新型催化不对称转化的催化剂负载量为 0.2 至 5.0 mol%,可以轻松、高产地获得高度对映体富集的手性三氟甲基化 γ-氨基酸和 γ-内酰胺。
更新日期:2018-01-18
中文翻译:

通过三氟甲基亚胺与羧酸衍生物的 Umpolung 加成催化不对称合成三氟甲基化 γ-氨基酸
新型金鸡纳生物碱衍生的手性相转移催化剂能够实现三氟甲基亚胺与α,β-不饱和N-酰基吡咯的高度化学、区域、非对映和对映选择性反极性加成。这种新型催化不对称转化的催化剂负载量为 0.2 至 5.0 mol%,可以轻松、高产地获得高度对映体富集的手性三氟甲基化 γ-氨基酸和 γ-内酰胺。



























 京公网安备 11010802027423号
京公网安备 11010802027423号