Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2017-11-23 , DOI: 10.1016/j.bioorg.2017.11.016 Fatima Ahsan 1 , Qurratulann Afza Gardner 1 , Naeem Rashid 1 , Greg J Towers 2 , Muhammad Akhtar 3
|
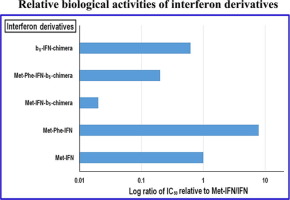
We have previously shown that human interferon α-2b (IFN) produced in Escherichia coli (E. coli) is heterogeneous at the N-terminal, with three major species (Ahsan et al., 2014). These are: (a) the direct translation product of the gene retaining the N-terminal methionine, (b) a species from which the methionyl residue has been removed by E. coli methionyl aminopeptidase to give the native interferon α-2b and (c) in which the N-terminal Cys residue of the latter contains an acetyl group. In this paper we overcome this heterogeneity, using engineered interferon derivatives with phenylalanine residue directly downstream of the N-terminal methionine (Met-Phe-IFN). This modification not only prevented the removal of the N-terminal methionine by E. coli methionyl aminopeptidase but also the subsequent N-acetylation. Critically, Met-Phe-IFN had enhanced activity in a biological assay. N-terminal stabilization was also achieved by fusing human cytochrome b5 at the N-terminal of interferon (b5-IFN-chimera). In this case also, the protein was more active than a reciprocal chimera with cytochrome b5 at the C-terminal of interferon (Met-IFN-b5-chimera). This latter protein also had a heterogeneous N-terminal but addition of phenylalanine following Met, (Met-Phe-IFN-b5-chimera), resolved this problem and gave enhanced biological activity.
中文翻译:

阻止人干扰素α-2b及其在大肠杆菌中表达的嵌合衍生物的N端加工
我们之前已经表明,大肠杆菌( E. coli )产生的人干扰素 α-2b (IFN)在 N 端是异质的,主要有三个种类 (Ahsan et al., 2014)。它们是:(a) 保留 N-末端甲硫氨酸的基因的直接翻译产物,(b) 甲硫氨酰残基已被大肠杆菌甲硫氨酰氨肽酶去除以产生天然干扰素 α-2b 的物种和(c ),其中后者的 N 端 Cys 残基含有乙酰基。在本文中,我们克服了这种异质性,使用在 N 末端甲硫氨酸 (Met-Phe-IFN) 下游直接带有苯丙氨酸残基的工程化干扰素衍生物。这种修饰不仅阻止了 N 末端甲硫氨酸的去除大肠杆菌的甲硫氨酰氨基肽酶也可随后进行N-乙酰化。至关重要的是,Met-Phe-IFN 在生物测定中具有增强的活性。N-末端稳定也通过在干扰素的N-末端融合人细胞色素b 5 (b 5 -IFN-嵌合体)来实现。在这种情况下,该蛋白质也比在干扰素的 C 末端具有细胞色素 b 5的相互嵌合体(Met-IFN-b 5 -嵌合体)更具活性。后一种蛋白质也有异质的 N 端,但在 Met 之后添加苯丙氨酸(Met-Phe-IFN-b 5 -嵌合体)解决了这个问题并增强了生物活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号